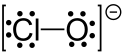Hypochlorite
[2] The name can also refer to esters of hypochlorous acid, namely organic compounds with a ClO– group covalently bound to the rest of the molecule.
A lowered pH (i.e. towards acid) drives the following reaction to the right, liberating chlorine gas, which can be dangerous: Hypochlorites are generally unstable and many compounds exist only in solution.
[citation needed] Upon heating, hypochlorite degrades to a mixture of chloride, oxygen, and chlorates: This reaction is exothermic and in the case of concentrated hypochlorites, such as LiOCl and Ca(OCl)2, can lead to dangerous thermal runaway and is potentially explosive.
[1] Hypochlorite salts are formed by the reaction between chlorine and alkali and alkaline earth metal hydroxides.
In response to infection, the human immune system generates minute quantities of hypochlorite within special white blood cells, called neutrophil granulocytes.
[12] These granulocytes engulf viruses and bacteria in an intracellular vacuole called the phagosome, where they are digested.
Superoxide decays to oxygen and hydrogen peroxide, which is used in a myeloperoxidase-catalysed reaction to convert chloride to hypochlorite.
[13][14][15] Low concentrations of hypochlorite were also found to interact with a microbe's heat shock proteins, stimulating their role as intra-cellular chaperone and causing the bacteria to form into clumps that will eventually die off.
[16] The same study found that low (micromolar) hypochlorite levels induce E. coli and Vibrio cholerae to activate a protective mechanism, although its implications were not clear.
They were the first commercial bleaching products, developed soon after that property was discovered in 1785 by French chemist Claude Berthollet.
That application started soon after French chemist Labarraque discovered those properties, around 1820 (still before Pasteur formulated his germ theory of disease).
This can be seen by comparing the standard half cell potentials across the series; the data also shows that the chlorine oxyanions are stronger oxidizers in acidic conditions.