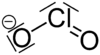
Chlorite
The chlorite ion, or chlorine dioxide anion, is the halite with the chemical formula of ClO−2.
Heavy metal chlorites (Ag+, Hg+, Tl+, Pb2+, and also Cu2+ and NH+4) are unstable and decompose explosively with heat or shock.
[1] Chlorite is the strongest oxidiser of the chlorine oxyanions on the basis of standard half cell potentials.
However, despite its strongly oxidizing nature, it is often not used directly, being instead used to generate the neutral species chlorine dioxide (ClO2), normally via a reaction with HCl: In 2009, the California Office of Environmental Health Hazard Assessment, or OEHHA, released a public health goal of maintaining amounts lower than 50 parts per billion for chlorite in drinking water[3] after scientists in the state reported that exposure to higher levels of chlorite affect sperm and thyroid function, cause stomach ulcers, and caused red blood cell damage in laboratory animals.
[6] Several oxyanions of chlorine exist, in which it can assume oxidation states of −1, +1, +3, +5, or +7 within the corresponding anions Cl−, ClO−, ClO−2, ClO−3, or ClO−4, known commonly and respectively as chloride, hypochlorite, chlorite, chlorate, and perchlorate.