IRAK1
IRAK-1 is part of the IRAK family consisting of IRAK-1, IRAK-2, IRAK-3, and IRAK-4, and is activated by inflammatory molecules released by signaling pathways during pathogenic attack.
[7] IRAK-1 is classified as a kinase enzyme, which regulates pathways in both innate and adaptive immune systems.
The proST domain contains serine, proline, and threonine amino acid residues and is used to facilitate IRAK-1 interaction with other IRAK family members or proteins.
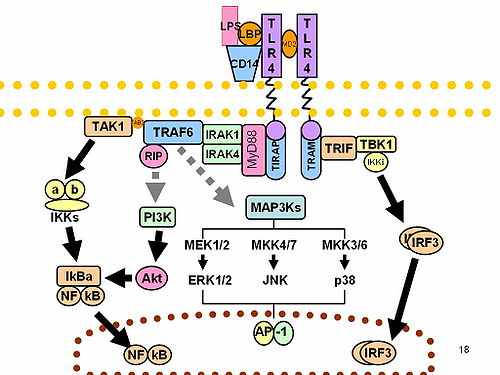
For example, auto-phosphorylation may occur multiple times in the ProST domain, which allows IRAK-1 to dissociate from the MyD88 bound to the DD while maintaining interactions with downstream proteins such as TNF receptor-associated factor 6 (TRAF-6) to initiate further pathway signaling.
The invariant lysine acts as a binding site for ATP and a mediator for catalytic function and kinase activity.
TAK-1 is then activated and phosphorylation of the inhibitor of κB kinase (IKK) complex, consisting of IKKα, IKKβ, and IKKγ, occurs.
IRAK1c, notably, remains stable upon sumoylation, does not undergo modification under the same circumstances and localizes only to the cytoplasm.
Moreover, IRAK-1c splice variant has a truncated and thus mutated sequence at the C-terminus of its kinase domain and acts a negative regulator of the TLR and IL-1R signaling pathways.