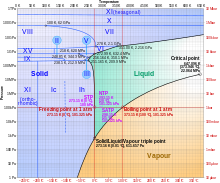
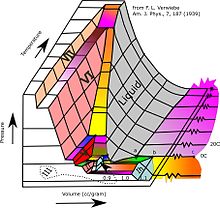
Phases of ice
In modern history, phases have been discovered through scientific research with various techniques including pressurization, force application, nucleation agents, and others.
The crystal lattice allows a substantial amount of disorder in the positions of the hydrogen atoms frozen into the structure as it cools to absolute zero.
More complex methods can be employed to better approximate the exact number of possible configurations, and achieve results closer to measured values.
According to the DFC calculation by Nakamura et al.,[84] the phase boundary between ice II and its disordered counterpart is estimated to be in the stability region of liquid water.
1981 research by Engelhardt and Kamb elucidated crystal structure of ice IV through a low-temperature single-crystal X-ray diffraction, describing it as a rhombohedral unit cell with a space group of R-3c.
[87] and Raman [88] The disordered nature of Ice IV was confirmed by neutron powder diffraction studies by Lobban (1998) [89] and Klotz et al.
2011 research by Salzmann's group reported more detailed DSC data where the endothermic feature becomes larger as the sample is quench-recovered at higher pressure.
[99] Hints of hydrogen-ordering in ice had been observed as early as 1964, when Dengel et al. attributed a peak in thermo-stimulated depolarization (TSD) current to the existence of a proton-ordered ferroelectric phase.
[100] However, they could not conclusively prove that a phase transition had taken place, and Onsager pointed out that the peak could also arise from the movement of defects and lattice imperfections.
The material had a polarization that had a decay length of 30 monolayers suggesting that thin layers of ice XI can be grown on substrates at low temperature without the use of dopants.
Some researchers suggested that, in combination with density functional theory calculations, none of the possible perfectly ordered orientational configurations are energetically favoured.
[119] Other researchers argued that P-1 model is still the best (with the second best candidate of P21), whereas Rietveld refinement using the Pmmn space group only works well for poorly ordered samples.
[79] In 2005 Laurence Fried led a team at Lawrence Livermore National Laboratory (LLNL) to recreate the formative conditions of superionic water.
Using a technique involving smashing water molecules between diamonds and super heating it with lasers they observed frequency shifts which indicated that a phase transition had taken place.
[129] In 2013 Hugh F. Wilson, Michael L. Wong, and Burkhard Militzer at the University of California, Berkeley published a paper predicting the face-centered cubic lattice structure that would emerge at higher pressures.
[131] More recent experiments from the same LLNL team used x-ray crystallography on laser-shocked water droplets to determine that the oxygen ions enter a face-centered-cubic phase, which was dubbed ice XVIII and reported in the journal Nature in May 2019.
In 2019, Alexander Rosu-Finsen and Christoph Salzman argued that there was no need to consider this to be a new phase of ice, and proposed a "deep-glassy" state scenario.
They concluded that the low-temperature endotherm originated from kinetic features related to glass transitions of deep glassy states of disordered ice VI.
Distinguishing between the two scenarios (new hydrogen-ordered phase vs. deep-glassy disordered ice VI) became an open question and the debate between the two groups has continued.
They found that the sign of the slope of the boundary turns negative from positive at 1.6 GPa indicating the existence of two different phases by the Clausius–Clapeyron relation.
Gasser et al.[136] also collected powder neutron diffractograms of quench-recovered ices VI, XV, and XIX and found similar crystallographic features to those reported by Yamane et al., concluding that P-4 and Pcc2 are the plausible space group candidates.
Amorphous ice may also form in the coldest region of the Earth's atmosphere, the summer polar mesosphere, where noctilucent clouds exist.
[166] These low temperatures are readily achieved in astrophysical environments such as molecular clouds, circumstellar disks, and the surfaces of objects in the outer Solar System.
Conversely, amorphous ice can be formed at temperatures higher than expected if the water flux is high, such as flash-freezing events associated with cryovolcanism.
[163] Peter Jenniskens and David F. Blake demonstrated in 1994 that a form of high-density amorphous ice is also created during vapor deposition of water on low-temperature (< 30 K) surfaces such as interstellar grains.
[170] When molecular clouds collapse to form stars, the temperature of the resulting circumstellar disk isn't expected to rise above 120 K, indicating that the majority of the ice should remain in an amorphous state.
If the original amorphous ice survived the molecular cloud collapse, then it should have been preserved at heliocentric distances beyond Saturn's orbit (~12 AU).
The amorphous ice might be explained by flash freezing from cryovolcanism, rapid condensation of molecules from water geysers, or irradiation of high-energy particles from Saturn.
[185] Ice VII may comprise the ocean floor of Europa as well as extrasolar planets (such as Awohali, and Enaiposha) that are largely made of water.
[195] On the other hand, there are also studies that suggest that other elements present inside the interiors of these planets, particularly carbon, may prevent the formation of superionic water.