Interferon beta-1a
[13] Treatment with interferons after an initial attack decreases the risk of developing clinical definite MS.[14][15] Medications are modestly effective at decreasing the number of attacks in relapsing-remitting multiple sclerosis[16] and in reducing the accumulation of brain lesions, which is measured using gadolinium-enhanced magnetic resonance imaging (MRI).
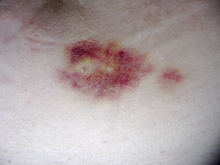
Skin reactions with interferon beta are more common with subcutaneous administration and vary greatly in their clinical presentation.
[20] Mild skin reactions usually do not impede treatment whereas necroses appear in around 5% of patients and lead to the discontinuation of the therapy.
[20] Also over time, a visible dent at the injection site due to the local destruction of fat tissue, known as lipoatrophy, may develop, however, this rarely occurs with interferon treatment.
[14][20] This reaction tends to disappear after 3 months of treatment and its symptoms can be treated with over-the-counter nonsteroidal anti-inflammatory drugs, such as ibuprofen, that reduce fever and pain.
[20] Interferon-beta can also reduce numbers of white blood cells (leukopenia), lymphocytes (lymphopenia) and neutrophils (neutropenia), as well as affect liver function.
[20] Nevertheless, recommendation is that all patients should be monitored through laboratory blood analyses, including liver function tests, to ensure safe use of interferons.
Side effects are often onerous enough that many patients ultimately discontinue taking interferons [citation needed] (or glatiramer acetate, a comparable disease-modifying therapy requiring regular injections).
[23] In vitro, interferon beta reduces production of Th17 cells which are a subset of T lymphocytes believed to have a role in the pathophysiology of MS.[24] Avonex was approved in the US in 1996,[25] and in the European Union in 1997, and is registered in more than 80 countries worldwide.
[citation needed] It is produced by the Biogen biotechnology company, originally under competition protection in the US under the Orphan Drug Act.
Avonex is sold in three formulations, a lyophilized powder requiring reconstitution, a pre-mixed liquid syringe kit, and a pen; it is administered via intramuscular injection.
[31] As of 2020, the National Average Drug Acquisition Cost (NADAC) in the United States for Avonex was $6,872.94 for a 30 mcg kit.
[34] Interferon beta-1a administered subcutaneously or intravenously was investigated since March 2020 as a potential treatment in patients hospitalized with COVID-19 in a multinational Solidarity trial (initially in combination with lopinavir) but it did not reduce in-hospital mortality compared to local standard of care.