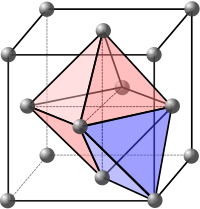
Interstitial site
In crystallography, interstitial sites, holes or voids are the empty space that exists between the packing of atoms (spheres) in the crystal structure.
[citation needed] The holes are easy to see if you try to pack circles together; no matter how close you get them or how you arrange them, you will have empty space in between.
This results in different shaped interstitial sites depending on the arrangement of the atoms in the lattice.
Looking at the three green spheres in the hexagonal packing illustration at the top of the page, they form a triangle-shaped hole.
If these voids are occupied by ions of opposite charge from the parent lattice, the cesium chloride structure is formed.