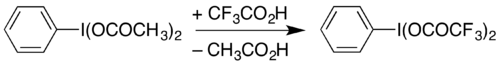(Diacetoxyiodo)benzene
(Diacetoxyiodo)benzene, also known as phenyliodine(III) diacetate (PIDA) is a hypervalent iodine chemical with the formula C6H5I(OCOCH3)2.
This reagent was originally prepared by Conrad Willgerodt[3] by reacting iodobenzene with a mixture of acetic acid and peracetic acid:[4][5] PIDA can also be prepared from iodosobenzene and glacial acetic acid:[5] More recent preparations direct from iodine, acetic acid, and benzene have been reported, using either sodium perborate[6] or potassium peroxydisulfate[7] as the oxidizing agent:[8] The PIDA molecule is termed hypervalent as its iodine atom (technically a hypervalent iodine) is in its +III oxidation state and has more than typical number of covalent bonds.
This second crystal structure determination explained the distortion in the geometry by noting the presence of two weaker intramolecular iodine–oxygen interactions, resulting in an "overall geometry of each iodine [that] can be described as a pentagonal-planar arrangement of three strong and two weak secondary bonds.
For example, it can be used to prepare (bis(trifluoroacetoxy)iodo)benzene (phenyliodine(III) bis(trifluoroacetate), PIFA) by heating in trifluoroacetic acid:[10][8] PIFA can be used to carry out the Hofmann rearrangement under mildly acidic conditions,[11] rather than the strongly basic conditions traditionally used.
[12][13] The Hofmann decarbonylation of an N-protected asparagine has been demonstrated with PIDA, providing a route to β-amino-L-alanine derivatives.