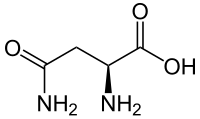
Asparagine
It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid.
[18] Since the asparagine side-chain can form hydrogen bond interactions with the peptide backbone, asparagine residues are often found near the beginning of alpha-helices as asx turns and asx motifs, and in similar turn motifs, or as amide rings, in beta sheets.
Typically, a carbohydrate tree can solely be added to an asparagine residue if the latter is flanked on the C side by X-serine or X-threonine, where X is any amino acid with the exception of proline.
[20] Asparagine is not essential for humans, which means that it can be synthesized from central metabolic pathway intermediates and is not required in the diet.
Glutamine donates an ammonium group, which reacts with β-aspartyl-AMP to form asparagine and free AMP.
[21] Heating a mixture of asparagine and reducing sugars or other source of carbonyls produces acrylamide in food.
These products occur in baked goods such as French fries, potato chips, and toasted bread.