Ion-exchange resin
[1] It is an insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radius) microbeads, usually white or yellowish, fabricated from an organic polymer substrate.
There are multiple types of ion-exchange resin, that differ in composition if the target is an anion or a cation.
In many cases, ion-exchange resins were introduced in such processes as a more flexible alternative to the use of natural or artificial zeolites.
This, combined with a high rate of ion exchange, make weakly base anion resins well suited for the organic salts.
[6] Reaction: Anion-exchange chromatography makes use of this principle to extract and purify materials from mixtures or solutions.
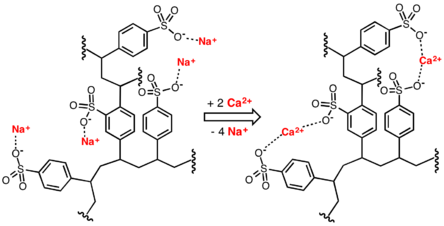
This process reaches equilibrium with a much lower concentration of magnesium and calcium ions in solution than was started with.
The resin can be recharged by washing it with a solution containing a high concentration of sodium ions (e.g. it has large amounts of common salt (NaCl) dissolved in it).
Water of highest purity is required for electronics, scientific experiments, production of superconductors, and nuclear industry, among others.
Ion exchange was for many years the only practical way to separate the rare earths in large quantities.
Then, the plutonium and uranium are available for making nuclear-energy materials, such as new reactor fuel and nuclear weapons.
In-situ recovery involves the extraction of uranium-bearing water (grading as low as 0.05% U3O8) through boreholes.
The ion-exchange process is also used to separate other sets of very similar chemical elements, such as zirconium and hafnium, which incidentally is also very important for the nuclear industry.
Three ion-exchange resins, sodium polystyrene sulfonate, colestipol, and cholestyramine, are used as active ingredients.
Sodium polystyrene sulfonate is a strongly acidic ion-exchange resin and is used to treat hyperkalemia.
In these uses the ion-exchange resin can have several different functions, including taste-masking, extended release, tablet disintegration, increased bioavailability, and improving the chemical stability of the active ingredients.
Although this method has only a limited efficacy, unlike small-molecular chelators (deferasirox, deferiprone, or deferoxamine), such an approach may have only minor side effects in sub-chronic studies.
A prototype demonstrating this process has been developed by Klaus Lackner at the Center for Negative Carbon Emissions.