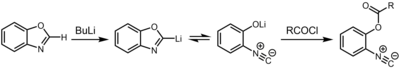Isocyanide
Lieke remarked that "Es besitzt einen penetranten, höchst unangenehmen Geruch; das Oeffnen eines Gefässes mit Cyanallyl [sic] reicht hin, die Luft eines Zimmers mehrere Tage lang zu verpesten [It has a penetrating, extremely unpleasant odour; the opening of a flask of allyl cyanide [sic] is enough to foul up the air in a room for several days]...."[6]: 319 Note that in Lieke's day, the difference between isocyanide and nitrile was not fully appreciated.
Ivar Karl Ugi states that "The development of the chemistry of isocyanides has probably suffered only little delay through the characteristic odor of volatile isonitriles, which has been described by Hofmann and Gautier as 'highly specific, almost overpowering', 'horrible', and 'extremely distressing'.
[8] Some isocyanides convey less offensive odours such as malt, natural rubber, creosote, cherry or old wood.
Referring to ethyl isocyanide, toxicological studies in the 1960s at Bayer showed that "oral and subcutaneous doses of 500-5000 mg/kg can be tolerated by mice".
The formamide can be dehydrated with toluenesulfonyl chloride, phosphorus oxychloride, phosgene, diphosgene, or the Burgess reagent in the presence of a base such as pyridine or triethylamine.
[16] In the carbylamine reaction (also known as the Hofmann isocyanide synthesis) alkali base reacts with chloroform to produce dichlorocarbene.
Illustrative is the synthesis of tert-butyl isocyanide from tert-butylamine in the presence of catalytic amount of the phase transfer catalyst benzyltriethylammonium chloride.
[9] The resulting organolithium compound exists in chemical equilibrium with the 2-isocyanophenolate, which can be captured by an electrophile such as an acid chloride.
[20] They also undergo insertion into the C–Cl bonds of acyl chlorides in the Nef isocyanide reaction, a process that is believed to be concerted and illustrates their carbene character.
Isocyanides have also been shown to be a useful reagent in palladium catalysed reactions with a wide variety of compounds being formed using this method.
[25] Although structurally similar, the analogous carbonyls differ in several ways, mainly because t-BuNC is a better donor ligand than CO.
[27] IUPAC uses the prefix "isocyano" for the systematic nomenclature of isocyanides: isocyanomethane, isocyanoethane, isocyanopropane, etc.