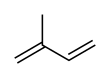
Isoprene
The first one to observe recombination of isoprene into rubber-like substance was Gustave Bouchardat [de] in 1879, and William A. Tilden identified its structure five years later.
Yearly production of isoprene emissions by vegetation is around 600 million metric tons, half from tropical broadleaf trees and the remainder primarily from shrubs.
[7][8][9] The estimated production rate of isoprene in the human body is 0.15 μmol/(kg·h), equivalent to approximately 17 mg/day for a person weighing 70 kg.
Human breath isoprene originates from lipolytic cholesterol metabolism within the skeletal muscular peroxisomes and IDI2 gene acts as the production determinant.
Many species of soil and marine bacteria, such as Actinomycetota, are capable of degrading isoprene and using it as a fuel source.
Thus, during the night, little isoprene is emitted from tree leaves, whereas daytime emissions are expected to be substantial during hot and sunny days, up to 25 μg/(g dry-leaf-weight)/hour in many oak species.
Heme A has an isoprenoid tail, and lanosterol, the sterol precursor in animals, is derived from squalene and hence from isoprene.
The functional isoprene units in biological systems are dimethylallyl pyrophosphate (DMAPP) and its isomer isopentenyl pyrophosphate (IPP), which are used in the biosynthesis of naturally occurring isoprenoids such as carotenoids, quinones, lanosterol derivatives (e.g. steroids) and the prenyl chains of certain compounds (e.g. phytol chain of chlorophyll).
Isoprenes are used in the cell membrane monolayer of many Archaea, filling the space between the diglycerol tetraether head groups.
Some natural rubber sources, called gutta percha, are composed of trans-1,4-polyisoprene, a structural isomer that has similar, but not identical, properties.