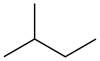
Isopentane
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon (an alkane) with five carbon atoms, with formula C5H12 or CH(CH3)2(C2H5).
[4] Although the mixture of pentanes was first isolated from the destructive distillation (pyrolysis) products of the boghead coal by Charles Greville Williams in 1862.
Carl Schorlemmer noted "that a mere trace of the liquid boiled below 30°C",[6] but the first to properly separate isomers (and thus discover isopentane) was American chemist Cyrus Warren (1824–1891) slightly later, who measured the boiling point of the more volatile one at 30°C.
Isopentane is used in a closed loop in geothermal power production to drive turbines.
[11] Isopentane is used, in conjunction with dry ice or liquid nitrogen, to freeze tissues for cryosectioning in histology.