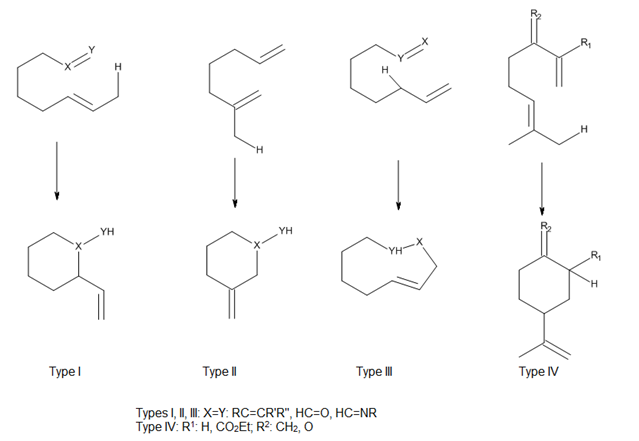
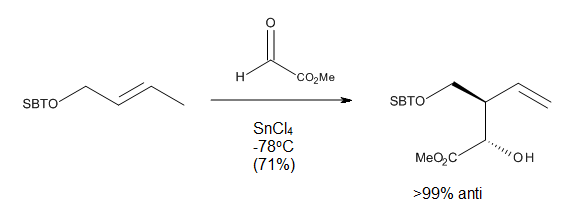
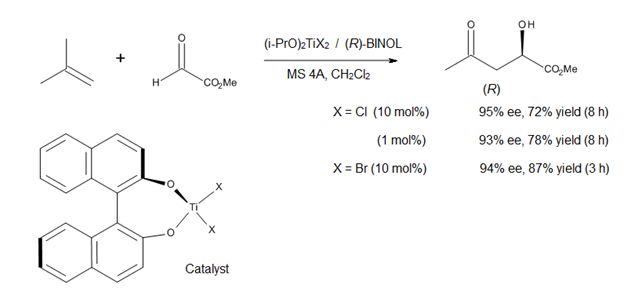
Ene reaction
For instance, kinetic data and computational studies indicate that thermolysis of but-3-enoic acid to give propene and carbon dioxide proceeds via a retro-ene mechanism.
[8] However, if the enophile becomes more polar (going from ethane to formaldehyde), its LUMO has a larger amplitude on C, yielding a better C–C overlap and a worse H–O one, determining the reaction to proceed in an asynchronous fashion.
[7] The early transition state proposed for the thermal ene reaction of propene with formaldehyde has an envelope conformation, with a C–O–H angle of 155°, as calculated at the 3-21G level of theory.
In thermal ene reactions, the order of reactivity for the abstracted H atom is primary> secondary> tertiary, irrespective of the thermodynamic stability of the internal olefin product.
In Lewis-acid promoted reactions, the pair enophile/Lewis acid employed determines largely the relative ease of abstraction of methyl vs. methylene hydrogens.
[2] The orientation of ene addition can be predicted from the relative stabilization of the developing partial charges in an unsymmetrical transition state with early formation of the σ bond.
[2] Intramolecular ene reactions benefit from less negative entropies of activation than their intermolecular counterparts, so are usually more facile, occurring even in the case of simple enophiles, such as unactivated alkenes and alkynes.
The reason behind the success of this catalyst is the fact that the ene-adduct- Me2AlCl complex can further react to afford methane and aluminum alkoxide, which can prevent proton-catalyzed rearrangements and solvolysis (Figure 9).
[3] In the case of directed carbonyl-ene reactions, high levels of regio- and stereo-selectivity have been observed upon addition of a Lewis acid, which can be explained through chair-like transition states.
The method affords α-hydroxy esters of high enantiomeric purities, compounds that represent a class of biological and synthetic importance (Figure 12).
Laulimalide is a marine natural product, a metabolite of various sponges that could find a potential use as an anti-tumor agent, due to its ability to stabilize microtubuli.
Treatment of the terminal allyl group of compound 1 with ethyl glyoxylate in the presence of catalytic (S)-BINOL-TiBr2 provided the required alcohol in 74% yield and >95% ds.
In addition, by carrying out this reaction, Pitts et al. managed to avoid the harsh conditions and low yields associated with installing exo-methylene units late in the synthesis.
The catalysts were found to afford high levels of asymmetric induction in several processes, including the ene reaction of ethyl glyoxylate with different unactivated olefins.
The reaction has a wide scope, as shown in Figure 16, owing to the high Lewis acidity of the catalysts, which can activate even weakly nucleophilic olefins, such as 1-hexene and cyclohexene.
[17] The azide displacement of the alcohol that results from the carbonyl ene reaction provides a facile route towards the synthesis of orthogonally protected amino acids.
The synthetic utility of the chiral C2-symmetric Cu(II) catalysts was truly revealed in the formation of the C17 stereocenter of the CD ring fragment of (+)-azaspiracid-1, a very potent toxin (cytotoxic to mammalian cells) produced in minute quantities by multiple shellfish species including mussels, oysters, scallops, clams, and cockles.