Isotope-ratio mass spectrometry
On the other hand, radiogenic isotope analysis[3] involves measuring the abundances of decay-products of natural radioactivity, and is used in most long-lived radiometric dating methods.
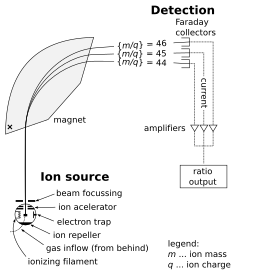
In the most general terms the instrument operates by ionizing the sample of interest, accelerating it over a potential in the kilo-volt range, and separating the resulting stream of ions according to their mass-to-charge ratio (m/z).
In a "multicollector" instrument, the ion collector typically has an array of Faraday cups, which allows the simultaneous detection of multiple isotopes.
Thus a sample of sea water will exhibit a quite detectable isotopic-ratio difference when compared to Antarctic snowfall.
Samples must be introduced to the mass spectrometer as pure gases, achieved through combustion, gas chromatographic feeds,[6] or chemical trapping.
When these isotope ratios are measured by TIMS, mass-dependent fractionation occurs as species are emitted by the hot filament.
Fractionation occurs due to the excitation of the sample and therefore must be corrected for accurate measurement of the isotope ratio.
It requires a stable power supply, and is suitable for species with a low ionization potential, such as strontium (Sr), and lead (Pb).
The hot filament reaches a temperature of less than 2500°C, leading to the inability to create atomic ions of species with a high ionization potential, such as osmium (Os), and tungsten (Hf-W).
Though the TIMS method can create molecular ions instead in this case, species with high ionization potential can be analyzed more effectively with MC-ICP-MS. An alternative approach used to measure the relative abundance of radiogenic isotopes when working with a solid surface is secondary-ion mass spectrometry (SIMS).
This assembly allows the secondary ions to be focused based on their kinetic energy and mass-charge ratio in order to be accurately collected using a series of Faraday cups.
Due to the inherent instability of the plasma, this limits the precision of ICP-MS with a quadrupole analyzer to around 1%, which is insufficient for most radiogenic isotope systems.
However, some systems (e.g. Hf-W and Lu-Hf) are difficult or impossible to analyse by TIMS, due to the high ionization potential of the elements involved.
The Ar-ICP produces an ion-beam with a large inherent kinetic energy distribution, which makes the design of the mass-spectrometer somewhat more complex than it is the case for conventional TIMS instruments.
First, different from Quadrupole ICP-MS systems, magnetic sector instruments have to operate with a higher acceleration potential (several 1000 V) in order to minimize the energy distribution of the ion beam.
For example, the decay rate of the radioisotope 14C is widely used to date organic materials, but this approach was once limited to relatively large samples no more than a few thousand years old.
[12] This process allows a mixture of compounds to be purified and analyzed continuously, which can decrease the analysis time by a factor of four.
[12] Moving wire IRMS is quite sensitive, and samples containing as little as 1 nanomole of carbon can yield precise (within 1‰) results.