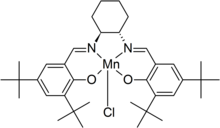Jacobsen's catalyst
Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand.
[6] The synthesis has been adapted for undergraduate level chemistry courses in order to stress the importance of enantiomerically pure compounds.
However, more recent studies have indicated a radical intermediate is possible, challenging the assumption that non-conjugated alkenes undergo concerted mechanisms.
[8] In the original catalytic reaction, iodosylarenes (PhIO) were used as the stoichiometric oxidant, but soon after it was found that chlorine bleach (NaClO), a cheaper alternative, works as well.
The substrate is thought to approach the metal-oxo bond from the side at a perpendicular orientation in relation to the catalyst in order to allow favorable orbital overlap.
This mechanism, which was originally proposed by John Groves to explain porphyrin-catalyzed epoxidation reactions,[9] is commonly referred to as a "side-on perpendicular approach".
[8] For example, derivatives of Jacobsen's catalyst with small structural changes to the salen backbone have been used in conjunction with low temperatures and the oxidant m-chloroperbenzoic acid (m-CPBA) to epoxidize the terminal alkene styrene.