Conjugated system
Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed.
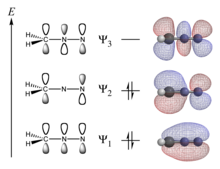
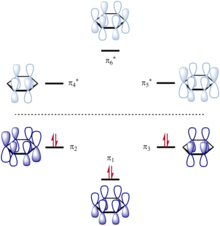
[2][a] A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond.
Conjugation is possible by means of alternating single and double bonds in which each atom supplies a p orbital perpendicular to the plane of the molecule.
For example, the delocalized π electrons in acetate anion and benzene are said to be involved in Π43 and Π66 systems, respectively (see the article on three-center four-electron bonding).
In compliance with the Pauli exclusion principle, overlapping p orbitals do not result in the formation of one large MO containing more than two electrons.
Hückel MO theory is commonly used approach to obtain a zeroth order picture of delocalized π molecular orbitals, including the mathematical sign of the wavefunction at various parts of the molecule and the locations of nodal planes.
It is particularly easy to apply for conjugated hydrocarbons and provides a reasonable approximation as long as the molecule is assumed to be planar with good overlap of p orbitals.
The quantitative estimation of stabilization from conjugation is notoriously contentious and depends on the implicit assumptions that are made when comparing reference systems or reactions.
Unambiguous examples are comparatively rare in neutral systems, due to a comparatively minor energetic benefit that is easily overridden by a variety of other factors; however, they are common in cationic systems in which a large energetic benefit can be derived from delocalization of positive charge (see the article on homoaromaticity for details.).
[14] Neutral systems generally require constrained geometries favoring interaction to produce significant degrees of homoconjugation.
[15] In the example below, the carbonyl stretching frequencies of the IR spectra of the respective compounds demonstrate homoconjugation, or lack thereof, in the neutral ground state molecules.
[16] Two appropriately aligned π systems whose ends meet at right angles can engage in spiroconjugation[17] or in homoconjugation across the spiro atom.
Vinylogy is the extension of a functional group through a conjugated organic bonding system, which transmits electronic effects.
Compounds that have a monocyclic, planar conjugated system containing (4n + 2) π-electrons for whole numbers n are aromatic and exhibit an unusual stability.
Because of the lack of long-range interactions, cyclooctatetraene takes on a nonplanar conformation and is nonaromatic in character, behaving as a typical alkene.
In contrast, derivatives of the cyclooctatetraene dication and dianion have been found to be planar experimentally, in accord with the prediction that they are stabilized aromatic systems with 6 and 10 π electrons, respectively.
[21] Many dyes make use of conjugated electron systems to absorb visible light, giving rise to strong colors.
A simple model of the energy levels is provided by the quantum-mechanical problem of a one-dimensional particle in a box of length L, representing the movement of a π electron along a long conjugated chain of carbon atoms.
For a chain of n C=C bonds or 2n carbon atoms in the molecular ground state, there are 2n π electrons occupying n molecular orbitals, so that the energy gap is[22] Since the box length L increases approximately linearly with the number of C=C bonds n, this means that the energy ΔE of a photon absorbed in the HOMO–LUMO transition is approximately proportional to 1/n.
[23] However, for good numerical agreement of the particle in a box model with experiment, the single-bond/double-bond bond length alternations of the polyenes must be taken into account.
With every double bond added, the system absorbs photons of longer wavelength (and lower energy), and the compound ranges from yellow to red in color.
This absorption of light in the ultraviolet to visible spectrum can be quantified using ultraviolet–visible spectroscopy, and forms the basis for the entire field of photochemistry.
Conjugated systems not only have low energy excitations in the visible spectral region but they also accept or donate electrons easily.
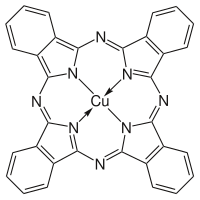
As a ligand, porphyrin forms numerous complexes with metallic ions like iron in hemoglobin that colors blood red.
Another similar macrocycle unit is corrin, which complexes with cobalt when forming part of cobalamin molecules, constituting Vitamin B12, which is intensely red.
Pdots are important labels for single-molecule fluorescence microscopy, based on high brightness, lack of blinking or dark fraction, and slow photobleaching.