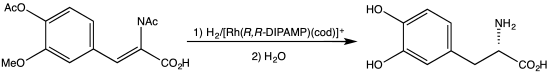
C2-Symmetric ligands
[1] The C2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues.
C2 symmetry improves the enantioselectivity of the complex by reducing the number of unique geometries in the transition states.
The carbonyl group will coordinate with the metal and due to the steric bulk of the phenyl group it will only be able to do so with its Si face exposed to the hydride ion with in the ideal situation exclusive formation of the (R) enantiomer.
Also note that if the steric bulk of both carbonyl substituents is very similar the strategy will fail.
Notably, EDTA and triethylenetetraamine form complexes that are C2-symmetric by virtue of the way the ligands wrap around the metal centers.
Ligands containing atomic chirality centers such asymmetric carbon, which usually do not have C2-symmetry, remain important in catalysis.