Ketene
Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group).
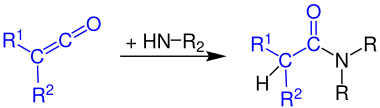
[4] Ketenes are highly electrophilic at the carbon atom bonded with the heteroatom, due to its sp character.
In the absence of nucleophiles with which to react, ethenone dimerises to give β-lactone, a cyclic ester.
Substituted ketenes can be prepared from acyl chlorides by an elimination reaction in which HCl is lost: In this reaction, a base, usually triethylamine, removes the acidic proton alpha to the carbonyl group, inducing the formation of the carbon-carbon double bond and the loss of a chloride ion:
Ketenes can also be formed from α-diazoketones by the Wolff rearrangement, and from vinylene carbonate by phosphorus(V) sulfide and irradiation.
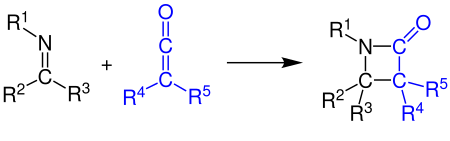
This system has a broad substrate scope and can be applied to various combinations of carbene precursors, nucleophiles and imines.
[1] A variety of hydroxylic compounds can add as nucleophiles, forming either enol or ester products.
Ethyl acetoacetate, an important starting material in organic synthesis, can be prepared using a diketene in reaction with ethanol.
They directly form ethyl acetoacetate, and the yield is high when carried out under controlled circumstances; this method is therefore used industrially.