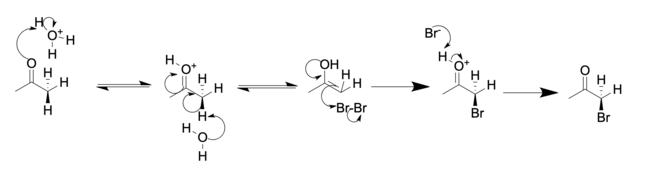
Ketone halogenation
In this way, chloride, bromide, and iodide (but notably not fluoride) functionality can be inserted selectively in the alpha position of a ketone.
This reaction undergoes a very reactive enol mechanism, facilitated by the CuO, which allows for the selective addition of I2 on the saturated alpha carbon of the ketone.
However, many of the current method for ketone halogenation use hazardous chemicals, have complex procedures, and/or require a long time to go to completion.
An experiment conducted by Meshram et al. in 2005 investigated making ketone halogenation a greener reaction, according to the principles of green chemistry.
[5][6] Meshram et al. investigated alternatives to the hazardous chemicals that are primarily used in ketone halogenation, finding that room temperature ionic liquids were a promising option.
Additionally, these ionic liquids have high polarity and their ability to solubilize organic and inorganic molecules leads to enhanced reaction rates, which makes them more desirable.