Kinetic isotope effect
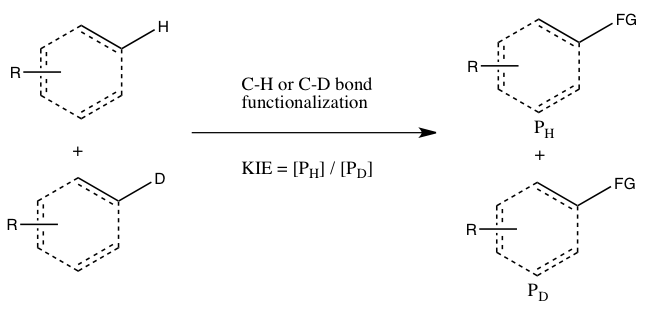
The study of KIEs can help elucidate reaction mechanisms, and is occasionally exploited in drug development to improve unfavorable pharmacokinetics by protecting metabolically vulnerable C-H bonds.
In many cases, the rate difference can be rationalized by noting that the mass of an atom affects the vibrational frequency of the chemical bond that it forms, even if the potential energy surface for the reaction is nearly identical.
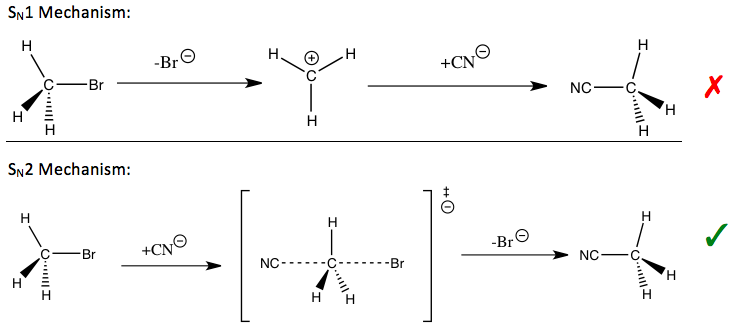
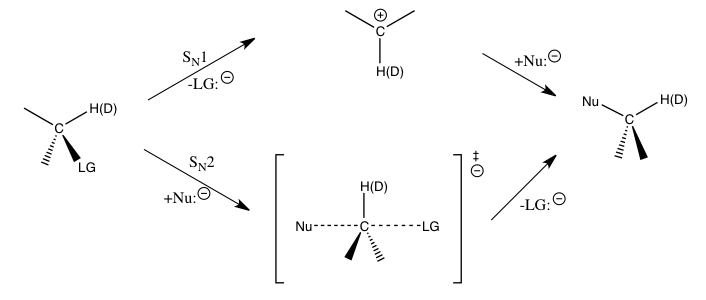
For an SN1 reaction, since the carbon atom is converted into an sp2 hybridized carbenium ion during the transition state for the rate-determining step with an increase in Cα-H(2H) bond order, an IKIE would be expected if only the stretching vibrations were important.
The observed large normal KIEs are found to be caused by significant out-of-plane bending vibrational contributions when going from the reactants to the transition state of carbenium ion formation.
Depending on the mass of the atom that moves along the reaction coordinate and nature (width and height) of the energy barrier, quantum tunneling may also make a large contribution to an observed KIE and may need to be separately considered, in addition to the "semi-classical" transition state theory model.
Moreover, several qualitative and semi-quantitative models allow rough estimates of deuterium isotope effects to be made without calculations, often providing enough information to rationalize experimental data or even support or refute different mechanistic possibilities.
In contrast, secondary effects are generally very small for heavier elements and close in magnitude to the experimental uncertainty, which complicates their interpretation and limits their utility.
It employs transition state theory and a statistical mechanical treatment of translational, rotational, and vibrational levels for the calculation of rate constants kH and kD.
[7] The complicated expression given above can be represented as the product of four separate factors:[7] For the special case of 2H isotope effects, we will argue that the first three terms can be treated as equal to or well approximated by unity.
Since hydrogen and deuterium tend to be much lighter than most reactants and transition states, there is little difference in the molecular masses and moments of inertia between H and D containing molecules, so the MMI factor is usually also approximated as unity.
Hence, the lower ZPE of the deuterated species translates into a larger activation energy for its reaction, as shown in the following figure, leading to a normal KIE.
)[15] Depending on the nature of the transition state of H-transfer (symmetric vs. "early" or "late" and linear vs. bent); the extent to which a primary 2H isotope effect approaches this maximum, varies.
Basing their arguments on transition state theory, the small A factor ratios associated with the large activation energy differences (usually about 4.5 kJ/mol for C–H(D) bonds) provided strong evidence for tunneling.
[30] Studies on ketosteroid isomerase have provided experimental evidence that the enzyme actually enhances the coupled motion/hydrogen tunneling by comparing primary and secondary KIEs of the reaction under enzyme-catalyzed and non-enzyme-catalyzed conditions.
Nevertheless, a measurement of a large kinetic isotope effect through direct comparison of rate constants is indicative that C-H bond cleavage occurs at the rate-determining step.
Pascal et al. were inspired by studies demonstrating dramatic variations of deuterium within identical compounds from different sources and hypothesized that NMR could be used to measure 2H KIEs at natural abundance.
[40] Singleton and coworkers demonstrated the capacity of 13C NMR based natural abundance KIE measurements for studying the mechanism of the [4 + 2] cycloaddition of isoprene with maleic anhydride.
[37] Previous studies by Gajewski on isotopically enrich materials observed KIE results that suggested an asynchronous transition state, but were always consistent, within error, for a perfectly synchronous reaction mechanism.
[37][clarification needed] Colletto et al. developed a regioselective β-arylation of benzo[b]thiophenes at room temperature with aryl iodides as coupling partners and sought to understand the mechanism of this reaction by performing natural abundance KIE measurements via single pulse NMR.
[42] Frost et al. sought to understand the effects of Lewis acid additives on the mechanism of enantioselective palladium-catalyzed C-N bond activation using natural abundance KIE measurements via single pulse NMR.
The addition of BPh3 causes a relative decrease in the observed 13C KIE which led Frost et al. to suggest a change in the rate limiting step from cis oxidation to coordination of palladium to the cyanoformamide.
To mitigate these limitations, Jacobsen and coworkers developed 1H to 13C polarization transfer as a means to reduce the time and material required for KIE measurements at natural abundance.
This method for natural abundance kinetic isotope measurement is favorable for analysis for reactions containing unstable starting materials, and catalysts or products that are relatively costly.
Jacobsen and coworkers observed small normal KIEs at C1, C2, and C5 which suggests significant oxocarbenium character in the transition state and an asynchronous reaction mechanism with a large degree of charge separation.
[46] The major limitation for determining KIEs at natural abundance using IRMS is the required site selective degradation without isotopic fractionation into an analyzable small molecule, a non-trivial task.
If the primary KIE is not as large, it is generally considered to be indicative of a significant contribution from heavy-atom motion to the reaction coordinate, though it may also mean that hydrogen transfer follows a nonlinear pathway.
[19] When carbon undergoes a reaction that changes its hybridization from sp3 to sp2, the out-of-plane bending force constant at the transition state is weaker as it is developing sp2 character and a "normal" SKIE is observed with typical values of 1.1 to 1.2.
[19] Conversely, when carbon's hybridization changes from sp2 to sp3, the out of plane bending force constants at the transition state increase and an inverse SKIE is observed with typical values of 0.8 to 0.9.
[48] An example of an "inverse" α SKIE can be seen in the work of Fitzpatrick and Kurtz who used such an effect to distinguish between two proposed pathways for the reaction of d-amino acid oxidase with nitroalkane anions.
[8] Similarly, combining nitrogen and hydrogen isotope effects was used to show that syn eliminations of simple ammonium salts also follow a concerted mechanism, which was a question of debate before.