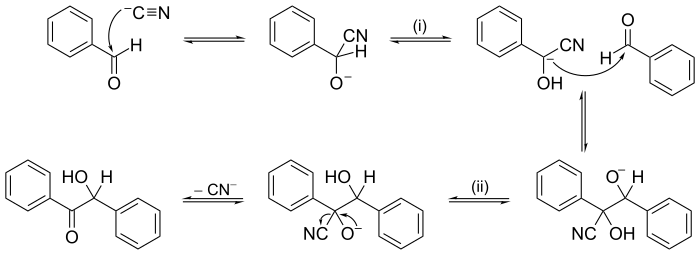
Reaction mechanism
The conjectured mechanism is chosen because it is thermodynamically feasible and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction.
A often quoted mechanistic experiment involved the reaction of the labile hexaaquo chromous reductant with the exchange inert pentammine cobalt(III) chloride.
The transition states are, in contrast, fleeting, high-energy species that cannot be isolated.
[2] Illustrative is the oxidation of carbon monoxide by nitrogen dioxide: The rate law for this reaction is:
A chain reaction is an example of a complex mechanism, in which the propagation steps form a closed cycle.
An example of a simple chain reaction is the thermal decomposition of acetaldehyde (CH3CHO) to methane (CH4) and carbon monoxide (CO).
The exact rate law may be even more complicated, there are also minor products such as acetone (CH3COCH3) and propanal (CH3CH2CHO).
For many combustion and plasma systems, detailed mechanisms are not available or require development.
Even when information is available, identifying and assembling the relevant data from a variety of sources, reconciling discrepant values and extrapolating to different conditions can be a difficult process without expert help.
Rate constants or thermochemical data are often not available in the literature, so computational chemistry techniques or group additivity methods must be used to obtain the required parameters.
Computational chemistry methods can also be used to calculate potential energy surfaces for reactions and determine probable mechanisms.
In general, reaction steps involving more than three molecular entities do not occur, because is statistically improbable in terms of Maxwell distribution to find such a transition state.