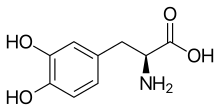
L-DOPA
l-DOPA, also known as l-3,4-dihydroxyphenylalanine and used medically as levodopa, is made and used as part of the normal biology of some plants[2] and animals, including humans.
[3][4] In some plant families (of the order Caryophyllales), l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains.
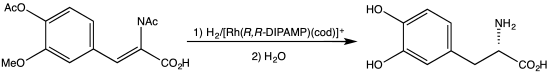
[5] l-DOPA can be manufactured and in its pure form is sold as a drug with the INNTooltip International Nonproprietary Name levodopa.
The enzyme tyrosinase catalyzes the oxidation of l-DOPA to the reactive intermediate dopaquinone, which reacts further, eventually leading to melanin oligomers.
In addition, tyrosinase can convert tyrosine directly to l-DOPA in the presence of a reducing agent such as ascorbic acid.
[12] l-dopa was first islolated from the seeds of the Vicia faba or broad bean plant in 1913 by Swiss biochemist Markus Guggenheim.
It is usually used in combination with a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor such as carbidopa or benserazide.