L-amino-acid oxidase
[2] In a similar vein, this enzyme performs in a myriad of biological activities including apoptosis-induction, edema-induction, hemorrhaging, and inhibition or induction of platelet aggregation.
[8] The substrate-binding site of the enzyme was determined to be at the base of a long funnel that extends 25 Å from the surface into the interior of the protein.
[8] It has also been determined that the FAD prosthetic group becomes deeply entrenched in the enzyme structure, which allows for pervasive interactions with both neighboring atoms and conserved water molecules.
[8] Additionally, this flavin-containing prosthetic group has been classified as providing snake venom with its quintessential dark yellow coloration, which is shown in Figure 2.
[16] In recent studies, it has been shown that LAAOs have been isolated from the skin and/or gill mucous secretions of rockfish, great sculpin, and flounder.
[17] The presence of these enzymes were identified to be a unique type of antibacterial protein in the external defense employed by certain fish species.
Notably, because of its potential in relevant antimicrobial, anti-tumor cell, and/or consumption of amino acids, the interest of researching sv-LAAOs has begun to grow.
[7] In one case study, it was reported that the sv-LAAO (isolated from C. durissus cascavella venom) caused the rupture of bacteria membranes while promoting extravasation, or leakage, of plasmatic contents out of the cellular structure.
While there are means of therapy to both prevent and cure cardiovascular diseases, many drugs are unavailable for clinical use due to severe side effects in addition to high toxicity levels.
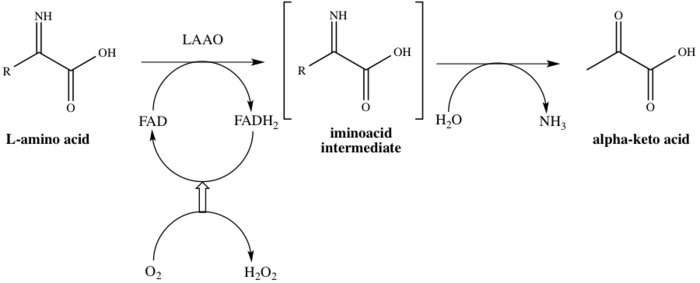
It has been proposed that hydrogen peroxide is considered to play a significant role regarding the enzymes ability to both cause and prevent this platelet aggregation.