Lamb shift
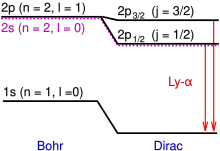
Hence the Lamb shift is a deviation from theory seen in the differing energies contained by the 2S1/2 and 2P1/2 orbitals of the hydrogen atom.
The Lamb shift is caused by interactions between the virtual photons created through vacuum energy fluctuations and the electron as it moves around the hydrogen nucleus in each of these two orbitals.
The Lamb shift has since played a significant role through vacuum energy fluctuations in theoretical prediction of Hawking radiation from black holes.
It was the harbinger of modern quantum electrodynamics developed by Julian Schwinger, Richard Feynman, Ernst Stueckelberg, Sin-Itiro Tomonaga and Freeman Dyson.
Victor Weisskopf regretted that his insecurity about his mathematical abilities may have cost him a Nobel Prize when he did not publish results (which turned out to be correct) about what is now known as the Lamb shift.
You were the first to see that this tiny shift, so elusive and hard to measure, would clarify our thinking about particles and fields.
The difference of potential energy is given by Since the fluctuations are isotropic, So one can obtain The classical equation of motion for the electron displacement (δr)k→ induced by a single mode of the field of wave vector k→ and frequency ν is and this is valid only when the frequency ν is greater than ν0 in the Bohr orbit,
But for s orbitals there is some finite value at the origin, where the Bohr radius is Therefore, Finally, the difference of the potential energy becomes: where
Welton's heuristic derivation of the Lamb shift is similar to, but distinct from, the calculation of the Darwin term using Zitterbewegung, a contribution to the fine structure that is of lower order in
[6]: 80–81 In 1947 Willis Lamb and Robert Retherford carried out an experiment using microwave techniques to stimulate radio-frequency transitions between 2S1/2 and 2P1/2 levels of hydrogen.
This particular difference is a one-loop effect of quantum electrodynamics, and can be interpreted as the influence of virtual photons that have been emitted and re-absorbed by the atom.
Thus, there exist small zero-point oscillations that cause the electron to execute rapid oscillatory motions.
The new potential can be approximated (using atomic units) as follows: The Lamb shift itself is given by with k(n, 0) around 13 varying slightly with n, and with log(k(n,ℓ)) a small number (approx.
For a derivation of ΔELamb see for example:[8] In 1947, Hans Bethe was the first to explain the Lamb shift in the hydrogen spectrum, and he thus laid the foundation for the modern development of quantum electrodynamics.
[9] The Lamb shift currently provides a measurement of the fine-structure constant α to better than one part in a million, allowing a precision test of quantum electrodynamics.