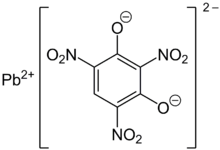
Lead styphnate
[3] Samples of lead styphnate vary in color from yellow to gold, orange, reddish-brown, to brown.
As with other lead-containing compounds, lead styphnate is toxic owing to heavy metal poisoning.
[6][3] Normal lead styphnate exists as α and β polymorphs, both being monoclinic crystals.
The styphnate ions lie in approximately parallel planes linked by Pb atoms.
[10] Lead styphnate is mainly used in small arms ammunition for military and commercial applications.