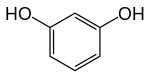
Resorcinol
Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide.
[6] Resorcinol is an expensive chemical, produced in only a very few locations around the world (as of 2010 only four commercial plants were known to be operative: in the United States, Germany, China, and Japan), and as such it is the determining factor in the cost of PRF adhesives.
[7] Production in the United States ended in 2017 with the closure of Indspec Chemical's plant in Petrolia, Pennsylvania.
Resorcinol can also be produced when any of a large number of resins (such as galbanum and asafoetida) are melted with potassium hydroxide, or by the distillation of Brazilwood extract.
[9] Many ortho- and para-compounds of the aromatic series (for example, the bromophenols, benzene-para-disulfonic acid) also yield resorcinol on fusion with potassium hydroxide.
Iron(III) chloride colors its aqueous solution a dark-violet, and bromine water precipitates tribromoresorcinol.
Sodium amalgam reduces it to dihydroresorcin, which when heated to 150 to 160 °C with concentrated barium hydroxide solution gives γ-acetylbutyric acid.
[13] With sodium nitrite it forms a water-soluble blue dye, which is turned red by acids, and is used as a pH indicator under the name of lacmoid.
[14] It condenses readily with aldehydes, yielding with formaldehyde, on the addition of catalytic hydrochloric acid, methylene diresorcin [(HO)C6H3(O)]2CH2.
Reaction with chloral hydrate in the presence of potassium bisulfate yields the lactone of tetra-oxydiphenyl methane carboxylic acid.
One such place where this treatment was given to soldiers with chronic acne was the Cambridge Military Hospital, Aldershot, England.
[22] Resazurin, C12H7NO4, obtained by the action of nitrous acid on resorcinol,[23] forms small dark red crystals possessing a greenish metallic glance.
Resorcinol is also a common scaffold that is found in a class of anticancer agents, some of which (luminespib, ganetespib, KW-2478, and onalespib) were in clinical trials as of 2014[update].
[25][26] Part of the resorcinol structure binds to inhibits the N-terminal domain of heat shock protein 90, which is a drug target for anticancer treatments.
[6] Resorcinol was named a substance of very high concern under European Union REACH in 2022 because of its endocrine disrupting properties.