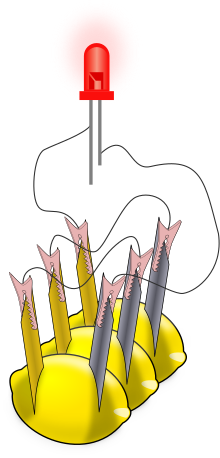
Lemon battery
There are many variations of the lemon cell that use different fruits (or liquids) as electrolytes and metals other than zinc and copper as electrodes.
There are numerous sets of instructions for making lemon batteries and for obtaining components such as light-emitting diodes, (LEDs), electrical meters (multimeters), and zinc-coated (galvanized) nails and screws.
For a more visible effect, lemon cells can be connected in series to power an LED (see illustration) or other devices.
Swartling and Morgan have published a list of low-voltage devices along with the corresponding number of lemon cells that were needed to power them; they included LEDs, piezoelectric buzzers, and small digital clocks.
Such a battery typically produces 0.001 A (1 mA) of current at a potential difference of 0.7 V; these values are multiplied together to determine the overall power of 0.0007 W (0.7 mW).
Other metals such as lead, iron, magnesium, etc., can be studied as well; they yield different voltages than the zinc/copper pair.
For older pupils and for college students, batteries serve to illustrate the principles of oxidation-reduction reactions.
This model of the chemical reactions makes several predictions that were examined in experiments published by Jerry Goodisman in 2001.
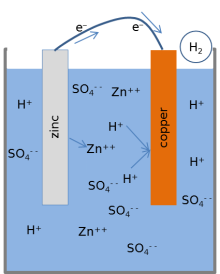
Goodisman notes that numerous recent authors propose chemical reactions for the lemon battery that involve dissolution of the copper electrode into the electrolyte.
When the electrolyte was modified by adding zinc sulfate (ZnSO4), the voltage from the cell was reduced as predicted using the Nernst equation for the model.
This result is consistent with the fact that copper atoms from the electrode are not involved in the chemical reaction model for the cell.
The two oxidation-reduction reactions listed above only occur when electrical charge can be transported through the external circuit.
From 1840 to the late 19th century, large, voltaic cells using a zinc electrode and a sulfuric acid electrolyte were widely used in the printing industry.
[18][19] Hydrogen gas clinging to the surface of a silver or copper electrode reduces the electric current that can be drawn from a cell; the phenomenon is called "polarization".
[17][20] The roughened, "platinized" surface speeds up the bubbling of the hydrogen gas, and increases the current from the cell.
The Smee cell was convenient for electrotyping, which produced copper plates for letterpress printing of newspapers and books, and also statues and other metallic objects.