Penny battery
The penny battery experiment is common during electrochemistry units in an educational setting.
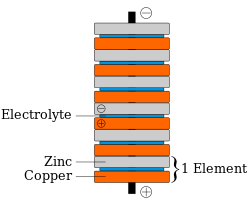
[3][4][5] A penny battery functions as a standard voltaic pile, and is powered by a redox reaction between zinc and acid.
The release of gas corresponds with a large increase in entropy, making the reaction irreversible.
Both types of cell can use acid as an electrolyte, copper as a cathode, and zinc as both an anode and as a substance to be oxidized.
Voltaic piles such as the penny battery start with all of their metal in solid form, so they don't contain any dissolved copper ions that can be reduced.