Lichexanthone
The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species.
In lichens, the biosynthesis of lichexanthone occurs through a set of enzymatic reactions that start with the molecule acetyl-CoA and sequentially add successive units, forming a longer chain that is cyclized into a double-ring structure.
[2] Asahina and Nogami used a chemical method called potash fusion (decomposition with a hot solution of the strong base potassium hydroxide) on lichexanthone to produce orcinol.
These enzymes control a number of enzymatic reactions through several coordinated active sites on a large multienzyme protein complex.
[25] The structure of lichen xanthones is derived by linear condensation of seven acetate and malonate units with one orsellinic acid-type cyclisation.
The two rings are joined by a ketonic carbon and by an ether-oxygen arising from cyclodehydration (i.e., a dehydration reaction leading to the formation of a cyclic compound).
[16] The exact mechanism is not known, but this ring closure might proceed through a benzophenone intermediate that could dehydrate to yield the central pyrone core of lichexanthone.
[26] The technique was later refined to couple the HPLC output with a photodiode array detector to screen for xanthones based on their specific ultraviolet–visible spectra.
In this way, lichexanthone is detected by monitoring its retention time, and verifying the presence of three peaks representing wavelengths of maximum absorption (λmax) at 208, 242, and 310 nm.
[28] The large genus Pertusaria relies heavily on thallus chemistry to distinguish and classify species, some of which differ only in the presence or absence of a single secondary chemical.
[20] In experiments using laboratory-grown mycobionts from the lichen Haematomma fluorescens, the synthesis of lichexanthone was induced when young mycelia were exposed to long-wavelength UV light (365 nm) for three to four hours every week over a time span of three to four months.
[49] The presence of the photoprotective chemical in the cortex may allow them to survive in otherwise inhospitable habitats, like on exposed trees in tropical areas or high mountains.
In some instances, similar or related species exist that lack cortical substances entirely, suggesting that the actual ecological function of lichexanthone is not fully understood.
According to the authors, this chemometrics approach was useful to correlate structural and chemical features with in vitro antimycobacterial activity among the group of ω-aminoalkoxylxanthones.
[19] Some authors have explicitly named lichexanthone in the specific epithets of their published lichen species, thereby acknowledging the presence of this compound as an important taxonomic characteristic.
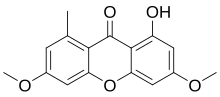




Me = methyl (–CH 3 )