Lithium
Like the other alkali metals (which are sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr)), lithium has a single valence electron that, in the presence of solvents, is easily released to form Li+.
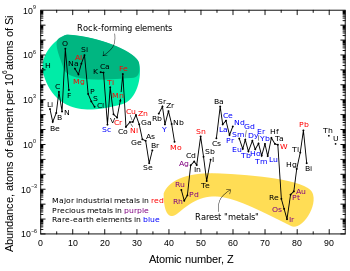
[30] Lithium isotopes fractionate substantially during a wide variety of natural processes,[31] including mineral formation (chemical precipitation), metabolism, and ion exchange.
Lithium ions substitute for magnesium and iron in octahedral sites in clay minerals, where 6Li is preferred to 7Li, resulting in enrichment of the light isotope in processes of hyperfiltration and rock alteration.
Granitic pegmatites also provide the greatest abundance of lithium-containing minerals, with spodumene and petalite being the most commercially viable sources.
There are a fairly large number of both lithium mineral and brine deposits but only comparatively few of them are of actual or potential commercial value.
[59] In December 2019, Finnish mining company Keliber Oy reported its Rapasaari lithium deposit has estimated proven and probable ore reserves of 5.280 million tonnes.
[60] In June 2010, The New York Times reported that American geologists were conducting ground surveys on dry salt lakes in western Afghanistan believing that large deposits of lithium are located there.
[68] Dry weight lithium concentrations for members of the family Solanaceae (which includes potatoes and tomatoes), for instance, can be as high as 30 ppm while this can be as low as 0.05 ppb for corn grains.
[68] Similarly, lithium at concentrations of 5 ppm reduces seed germination in some species (e.g. Asian rice and chickpea) but not in others (e.g. barley and wheat).
Petalite (LiAlSi4O10) was discovered in 1800 by the Brazilian chemist and statesman José Bonifácio de Andrada e Silva in a mine on the island of Utö, Sweden.
[80] Berzelius gave the alkaline material the name "lithion/lithina", from the Greek word λιθoς (transliterated as lithos, meaning "stone"), to reflect its discovery in a solid mineral, as opposed to potassium, which had been discovered in plant ashes, and sodium, which was known partly for its high abundance in animal blood.
After the end of the nuclear arms race, the demand for lithium decreased and the sale of department of energy stockpiles on the open market further reduced prices.
These clusters are broken down into smaller or monomeric units in the presence of solvents like dimethoxyethane (DME) or ligands like tetramethylethylenediamine (TMEDA).
This suggests that lithium will be one of the main objects of geopolitical competition, but this perspective has also been criticised for underestimating the power of economic incentives for expanded production.
Thus, the Democratic Republic of Congo is expected to be a significant supplier of lithium to the world with its high grade and low impurities.
[125] Similarly in Nevada, the McDermitt Caldera hosts lithium-bearing volcanic muds that consist of the largest known deposits of lithium within the United States.
[126] The Pampean Pegmatite Province in Argentina is known to have a total of at least 200,000 tons of spodumene with lithium oxide (Li2O) grades varying between 5 and 8 wt %.
The project aims to produce 45,000 tonnes of lithium carbonate and hydroxide per year and plans to reach full design capacity by 2030.
[131] After the 2007 financial crisis, major suppliers, such as Sociedad Química y Minera (SQM), dropped lithium carbonate pricing by 20%.
A 2012 Business Week article outlined an oligopoly in the lithium space: "SQM, controlled by billionaire Julio Ponce, is the second-largest, followed by Rockwood, which is backed by Henry Kravis's KKR & Co., and Philadelphia-based FMC", with Talison mentioned as the biggest producer.
[56] Large lithium-clay deposits under development in the McDermitt caldera (Nevada, United States) require concentrated sulfuric acid to leach lithium from the clay ore.[135] By early 2021, much of the lithium mined globally comes from either "spodumene, the mineral contained in hard rocks found in places such as Australia and North Carolina"[136] or from the salty brine pumped directly out of the ground, as it is in locations in Chile.
[137][138][139] One method for lithium extraction, as well as other valuable minerals, is to process geothermal brine water through an electrolytic cell, located within a membrane.
[146] Massive byproduct generation of lithium extraction also presents unsolved problems, such as large amounts of magnesium and lime waste.
A study of relationships between lithium extraction companies and indigenous peoples in Argentina indicated that the state may not have protected indigenous peoples' right to free prior and informed consent, and that extraction companies generally controlled community access to information and set the terms for discussion of the projects and benefit sharing.
[152] Development of the Thacker Pass lithium mine in Nevada, United States, has met with protests and lawsuits from several indigenous tribes who have said they were not provided free prior and informed consent and that the project threatens cultural and sacred sites.
Lithium oxide is widely used as a flux for processing silica, reducing the melting point and viscosity of the material and leading to glazes with improved physical properties including low coefficients of thermal expansion.
In the polymer industry, which is the dominant consumer of these reagents, alkyl lithium compounds are catalysts/initiators[181] in anionic polymerization of unfunctionalized olefins.
[182][183][184] For the production of fine chemicals, organolithium compounds function as strong bases and as reagents for the formation of carbon-carbon bonds.
[186] The Mark 50 torpedo stored chemical energy propulsion system (SCEPS) uses a small tank of sulfur hexafluoride, which is sprayed over a block of solid lithium.
[195][196] In 2013, the US Government Accountability Office said a shortage of lithium-7 critical to the operation of 65 out of 100 American nuclear reactors "places their ability to continue to provide electricity at some risk."