Low valent magnesium compounds
[2] The chemistry of Mg is dominated by the +2 oxidation state and prior to 2007 only examples of crystalline compounds with short Mg-Mg distances that may indicate an Mg-Mg bond were known, such as the ternary metal hydrides Mg2RuH4, Mg3RuH3, and Mg4IrH5 and magnesium diboride,[3] Calculations had also indicated the stability of the Mg22+ cation.
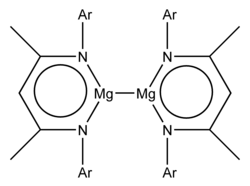
[4] The preparation of the first compounds made involved the reduction of MgII iodine complexes with potassium metal and the bulky ligands were:[2] Both examples have the formula L2Mg2, where L represents the bulky anionic bidentate ligand.
Since then a variety of stable Mg(I) compounds have been prepared, some melting over 200 °C, some colorless, others colored, but all involving very bulky ligands.
[1] Also complexes of the LMgMgL with monodentate ligands have been prepared and in these the coordination of the Mg atom increases from three to four.
[1] The magnesium(I) dimers have proved to be useful reducing agents, for example in the preparation of tin(I) compounds.