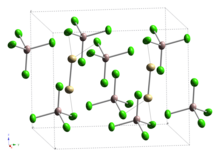
Cadmium(I) tetrachloroaluminate
It was the first compound reported (1961) that contained cadmium in the +1 oxidation state and features a cadmium–cadmium bond.
Cd2[AlCl4]2 was originally prepared by dissolving Cd metal in molten CdCl2 followed by the addition of AlCl3.
[2] Subsequent studies of the Raman vibrational spectrum indicated the presence of a cadmium–cadmium bond,[3] which was confirmed by two separate X-ray diffraction studies of single crystals.
The Cd−Cd single bonds are part of ethane-like Cd2Cl6 units sharing vertices with AlCl4 units, with a Cd−Cd bond length reported as 257.6 pm[4] or 256.1pm.
It contains no unpaired electrons and reacts readily with water disproportionating to give Cd metal and Cd2+.