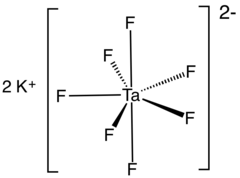
Potassium heptafluorotantalate
This white, water-soluble solid is an intermediate in the purification of tantalum from its ores and is the precursor to the metal.
[2] This solution is subjected to a number of liquid-liquid extraction steps to remove metallic impurities (most importantly niobium) before being treated with potassium fluoride to produce K2[TaF7] Hydrofluoric acid is both corrosive and toxic, making it unappealing to work with; as such a number of alternative processes have been developed for small-scale syntheses.
The K-salt can be also precipitated from a solution in hydrofluoric acid of tantalum pentachloride: Potassium heptafluorotantalate exists in at least two polymorphs.
In terms of the coordination sphere of the heavy metal, potassium heptafluoroniobate is similar to the tantalum salt.
In order to prevent hydrolysis and co-precipitation of potassium oxyfluorotantalate, a small excess of HF is added to the solution.