Macromolecule
The most common macromolecules in biochemistry are biopolymers (nucleic acids, proteins, and carbohydrates) and large non-polymeric molecules such as lipids, nanogels and macrocycles.
This statementfails in the case of certain macromolecules for which the properties may becritically dependent on fine details of the molecular structure.
[4] The term macromolecule (macro- + molecule) was coined by Nobel laureate Hermann Staudinger in the 1920s, although his first relevant publication on this field only mentions high molecular compounds (in excess of 1,000 atoms).
[5] At that time the term polymer, as introduced by Berzelius in 1832, had a different meaning from that of today: it simply was another form of isomerism for example with benzene and acetylene and had little to do with size.
[7] According to the standard IUPAC definition, the term macromolecule as used in polymer science refers only to a single molecule.
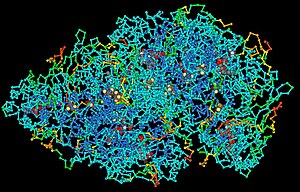
The structure of simple macromolecules, such as homopolymers, may be described in terms of the individual monomer subunit and total molecular mass.
Another common macromolecular property that does not characterize smaller molecules is their relative insolubility in water and similar solvents, instead forming colloids.
Therefore, they are not constrained by the regular geometry of the DNA double helix, and so fold into complex three-dimensional shapes dependent on their sequence.
These different shapes are responsible for many of the common properties of RNA and proteins, including the formation of specific binding pockets, and the ability to catalyse biochemical reactions.
DNA is an information storage macromolecule that encodes the complete set of instructions (the genome) that are required to assemble, maintain, and reproduce every living organism.
In addition, RNA is a single-stranded polymer that can, like proteins, fold into a very large number of three-dimensional structures.
[1]: 11 Because monosaccharides have multiple functional groups, polysaccharides can form linear polymers (e.g. cellulose) or complex branched structures (e.g. glycogen).
Polysaccharides perform numerous roles in living organisms, acting as energy stores (e.g. starch) and as structural components (e.g. chitin in arthropods and fungi).