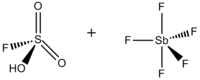Magic acid
This conjugate Brønsted–Lewis superacid system was developed in the 1960s by Ronald Gillespie and his team at McMaster University,[1] and has been used by George Olah to stabilise carbocations and hypercoordinated carbonium ions in liquid media.
Magic acid and other superacids are also used to catalyze isomerization of saturated hydrocarbons, and have been shown to protonate even weak bases, including methane, xenon, halogens, and molecular hydrogen.
[2] The term "superacid" was first used in 1927 when James Bryant Conant found that perchloric acid could protonate ketones and aldehydes to form salts in nonaqueous solution.
The name originated after a Christmas party in 1966, when a member of the Olah lab placed a paraffin candle into the acid, and found that it dissolved quite rapidly.
Examination of the solution with 1H-NMR showed a tert-butyl cation, suggesting that the paraffin chain that forms the wax had been cleaved, then isomerized into the relatively stable tertiary carbocation.
When the ratio SbF5:HSO3F is less than 0.2, the following two equilibria, determined by 19F NMR spectroscopy, are the most prominent in solution: (In both of these structures, the sulfur has tetrahedral coordination, not planar.
Due to the slow timescale of 1H-NMR, the rapidly equilibrating positive charges on hydrogen atoms would likely go undetected.
This is evidence to suggest that in these reactions, methane is indeed a base, and can accept a proton from the acid medium to form CH+5.
He says, "It is, however, with some nostalgia that we make this recommendation, as ‘inert gases’ at least maintained their ‘nobility’ as their chemical reactivity became apparent, but referring to ‘noble hydrocarbons’ would seem to be inappropriate.
The nature of the experiments used to determine the mechanism, namely the fact that they took place in superacid medium, allowed observation of the carbocation intermediates formed.
[8] Magic acid also catalyzes electrophilic hydroxylation of aromatic compounds with hydrogen peroxide, resulting in high-yield preparation of monohydroxylated products.
Phenols exist as completely protonated species in superacid solutions, and when produced in the reaction, are then deactivated toward further electrophilic attack.