Perchloric acid
French pharmacist Georges-Simon Serullas introduced the modern designation along with discovering its solid monohydrate, which he mistook for an anhydride.
The traditional method exploits the high aqueous solubility of sodium perchlorate (209 g/100 ml of water at room temperature).
The alternative route, which is more direct and avoids salts, entails anodic oxidation of aqueous chlorine at a platinum electrode.
[6] Perchloric acid is one of the most proven materials for etching of liquid crystal displays and critical electronics applications as well as ore extraction and has unique properties in analytical chemistry.
That its pKa is lower than −9 is evidenced by the fact that its monohydrate contains discrete hydronium ions and can be isolated as a stable, crystalline solid, formulated as [H3O+][ClO–4].
[15] Given its strong oxidizing properties, perchloric acid is subject to extensive regulations as it can react violently with metals and flammable substances such as wood, plastics, and oils.
On February 20, 1947 in Los Angeles, California, 17 people were killed and 150 injured in the O'Connor Plating Works disaster.
A bath, consisting of over 1000 litres of 75% perchloric acid and 35% acetic anhydride by volume which was being used to electro-polish aluminium furniture, exploded.
Organic compounds were added to the overheating bath when an iron rack was replaced with one coated with cellulose acetobutyrate (Tenit-2 plastic).
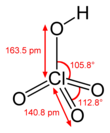
Hydroxidotrioxidochlorine

ydroxidotrioxidochlorine

