Magnesium hydride
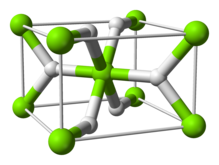
[4] Other preparations include: The room temperature form α-MgH2 has a rutile structure.
The bonding in the rutile form is sometimes described as being partially covalent in nature rather than purely ionic;[13] charge density determination by synchrotron x-ray diffraction indicates that the magnesium atom is fully ionised and spherical in shape and the hydride ion is elongated.
[14] Molecular forms of magnesium hydride, MgH, MgH2, Mg2H, Mg2H2, Mg2H3, and Mg2H4 molecules identified by their vibrational spectra have been found in matrix isolated samples at below 10 K, formed following laser ablation of magnesium in the presence of hydrogen.
[15] MgH2 readily reacts with water to form hydrogen gas: At 287 °C it decomposes to produce H2 at 1 bar pressure.
[16] The high temperature required is seen as a limitation in the use of MgH2 as a reversible hydrogen storage medium:[17]