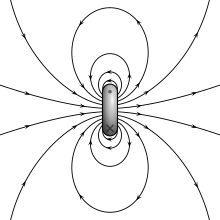
Magnetic moment
This definition is based on how one could, in principle, measure the magnetic moment of an unknown sample.
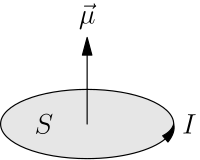
Further, this definition allows the calculation of the expected magnetic moment for any known macroscopic current distribution.
[5]: 23 In the CGS system, there are several different sets of electromagnetism units, of which the main ones are ESU, Gaussian, and EMU.
The ratio of these two non-equivalent CGS units (EMU/ESU) is equal to the speed of light in free space, expressed in cm⋅s−1.
Instead the parameter that is listed is residual flux density (or remanence), denoted Br.
Before the 1930s, textbooks explained the moment using hypothetical magnetic point charges.
[citation needed] The sources of magnetic moments in materials can be represented by poles in analogy to electrostatics.
This is valid for the moment due to any localized current distribution provided that the magnetic field is uniform.
In the case of a pair of monopoles being used (i.e. electric dipole model), the force is
Note that if there are no currents or time-varying electrical fields or magnetic charge, ∇×B = 0, ∇⋅B = 0 and the two expressions agree.
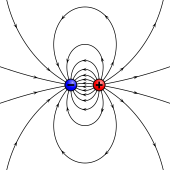
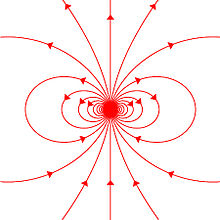
While the net magnetic field produced by the system can also have higher-order multipole components, those will drop off with distance more rapidly, so that only the dipole component will dominate the magnetic field of the system at distances far away from it.
The inverse cube nature of this equation is more readily seen by expressing the location vector
A magnetic dipole is the limit of either a current loop or a pair of poles as the dimensions of the source are reduced to zero while keeping the moment constant.
Both of these potentials can be calculated for any arbitrary current distribution (for the amperian loop model) or magnetic charge distribution (for the magnetic charge model) provided that these are limited to a small enough region to give:
This distinction only matters if the dipole limit is used to calculate fields inside a magnetic material.
Unlike the expressions in the previous section, this limit is correct for the internal field of the dipole.
[7][9]: 184 The magnetic moment has a close connection with angular momentum called the gyromagnetic effect.
The angular momentum of the very large number of charged particles that make up a current therefore is:
[16] This is similar to the magnetic moment created by the very large number of charged particles that make up that current:
For example, the g-factor for the magnetic moment due to an electron orbiting a nucleus is one while the g-factor for the magnetic moment of electron due to its intrinsic angular momentum (spin) is a little larger than 2.
In the atomic world the angular momentum (spin) of a particle is an integer (or half-integer in the case of fermions) multiple of the reduced Planck constant ħ.
Fundamentally, contributions to any system's magnetic moment may come from sources of two kinds: 1) motion of electric charges, such as electric currents; and 2) the intrinsic magnetism due spin of elementary particles, such as the electron.
The net magnetic moment of any system is a vector sum of contributions from one or both types of sources.
where j is the total angular momentum quantum number, gJ is the Landé g-factor, and μB is the Bohr magneton.
Electrons and many elementary particles also have intrinsic magnetic moments, an explanation of which requires a quantum mechanical treatment and relates to the intrinsic angular momentum of the particles as discussed in the article Electron magnetic moment.
where μB is the Bohr magneton, S is electron spin, and the g-factor gS is 2 according to Dirac's theory, but due to quantum electrodynamic effects it is slightly larger in reality: 2.00231930436.
Each energy state of a nucleus of a given isotope is characterized by a well-defined magnetic dipole moment, the magnitude of which is a fixed number, often measured experimentally to a great precision.
This number is very sensitive to the individual contributions from nucleons, and a measurement or prediction of its value can reveal important information about the content of the nuclear wave function.
There are several theoretical models that predict the value of the magnetic dipole moment and a number of experimental techniques aiming to carry out measurements in nuclei along the nuclear chart.
Typically, the overall magnetic moment of a molecule is a combination of the following contributions, in the order of their typical strength: In atomic and nuclear physics, the Greek symbol μ represents the magnitude of the magnetic moment, often measured in Bohr magnetons or nuclear magnetons, associated with the intrinsic spin of the particle and/or with the orbital motion of the particle in a system.