Maillard reaction
Seared steaks, fried dumplings, cookies and other kinds of biscuits, breads, toasted marshmallows, falafel and many other foods undergo this reaction.
[3] At higher temperatures, caramelization (the browning of sugars, a distinct process) and subsequently pyrolysis (final breakdown leading to burning and the development of acrid flavors) become more pronounced.
[7] In 1912, Louis Camille Maillard published a paper describing the reaction between amino acids and sugars at elevated temperatures.
It contributes to the darkened crust of baked goods, the golden-brown color of French fries and other crisps, browning of malted barley as found in malt whiskey and beer, and the color and taste of dried and condensed milk, dulce de leche, toffee, black garlic, chocolate, toasted marshmallows, and roasted peanuts.
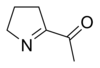
[citation needed] 6-Acetyl-2,3,4,5-tetrahydropyridine is responsible for the biscuit or cracker-like flavor present in baked goods such as bread, popcorn, and tortilla products.
[14] In making silage, excess heat causes the Maillard reaction to occur, which reduces the amount of energy and protein available to the animals that feed on it.
[18] Acrylamide, a possible human carcinogen,[19] can be generated as a byproduct of Maillard reaction between reducing sugars and amino acids, especially asparagine, both of which are present in most food products.