Manganese(II) acetate
Manganese(II) acetate are chemical compounds with the formula Mn(CH3CO2)2·(H2O)n where n = 0, 2, 4.
These materials are white or pale pink solids.
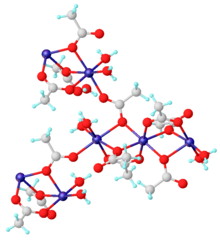
[3] Manganese(II) acetate can be formed by treating either manganese(II,III) oxide or manganese(II) carbonate with acetic acid:[4] The anhydrous material and dihydrate Mn(CH3CO2)2·2H2O are coordination polymers.
The dihydrate has been characterized by X-ray crystallography.
Each Mn(II) center is surrounded by six oxygen centers provided by aquo ligands and acetates.