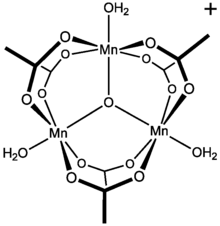
Manganese(III) acetate
Manganese(III) acetate describes a family of materials with the approximate formula Mn(O2CCH3)3.
These materials are brown solids that are soluble in acetic acid and water.
[3] This process is thought to proceed via the formation of a •CH2CO2H radical intermediate, which then reacts with the alkene, followed by additional oxidation steps and finally ring closure.
[7] It can similarly oxidize β-ketoesters at the α carbon, and this intermediate can react with various other structures, including halides and alkenes (see: manganese-mediated coupling reactions).
One extension of this idea is the cyclization of the ketoester portion of the molecule with an alkene elsewhere in the same structure.