Nuclear binding energy
Both the experimental and theoretical views are equivalent, with slightly different emphasis on what the binding energy means.
The Sun and other stars use nuclear fusion to generate thermal energy which is later radiated from the surface, a type of stellar nucleosynthesis.
A pair of protons would do 10−13 joules of work to each other as they approach – that is, they would need to release energy of 0.5 MeV in order to stick together.
The fact that nuclei do not clump together (fuse) under normal conditions suggests that the nuclear force must be weaker than the electric repulsion at larger distances, but stronger at close range.
The helium nucleus has four nucleons bound together, and the binding energy which holds them together is, in effect, the missing 0.8% of mass.
The nuclear fusion process works as follows: five billion years ago, the new Sun formed when gravity pulled together a vast cloud of hydrogen and dust, from which the Earth and other planets also arose.
The gravitational pull released energy and heated the early Sun, much in the way Helmholtz proposed.
[10] Thermal energy appears as the motion of atoms and molecules: the higher the temperature of a collection of particles, the greater is their velocity and the more violent are their collisions.
Instead, physicists use strong magnetic fields to confine the plasma, and for fuel they use heavy forms of hydrogen, which burn more easily.
Magnetic traps can be rather unstable, and any plasma hot enough and dense enough to undergo nuclear fusion tends to slip out of them after a short time.
[5] For elements that weigh more than iron (a nucleus with 26 protons), the fusion process no longer releases energy.
With such large nuclei, overcoming the electric repulsion (which affects all protons in the nucleus) requires more energy than is released by the nuclear attraction (which is effective mainly between close neighbors).
[12] The sun is composed of 74 percent hydrogen (measured by mass), an element having a nucleus consisting of a single proton.
Energy is released in the Sun when 4 protons combine into a helium nucleus, a process in which two of them are also converted to neutrons.
[11] The protons of hydrogen combine to helium only if they have enough velocity to overcome each other's mutual repulsion sufficiently to get within range of the strong nuclear attraction.
Hydrogen hot enough for combining to helium requires an enormous pressure to keep it confined, but suitable conditions exist in the central regions of the Sun, where such pressure is provided by the enormous weight of the layers above the core, pressed inwards by the Sun's strong gravity.
The Earth's oceans contain a large amount of deuterium that could be used and tritium can be made in the reactor itself from lithium, and furthermore the helium product does not harm the environment, so some consider nuclear fusion a good alternative to supply our energy needs.
However, in heavier nuclei, the disruptive energy of protons increases, since they are confined to a tiny volume and repel each other.
In the opposite process, which only happens within a nucleus, and not to free particles, a proton may become a neutron by ejecting a positron and an electron neutrino.
The heaviest nuclei in more than trace quantities in nature, uranium 238U, are unstable, but having a half-life of 4.5 billion years, close to the age of the Earth, they are still relatively abundant; they (and other nuclei heavier than helium) have formed in stellar evolution events like supernova explosions [14] preceding the formation of the Solar System.
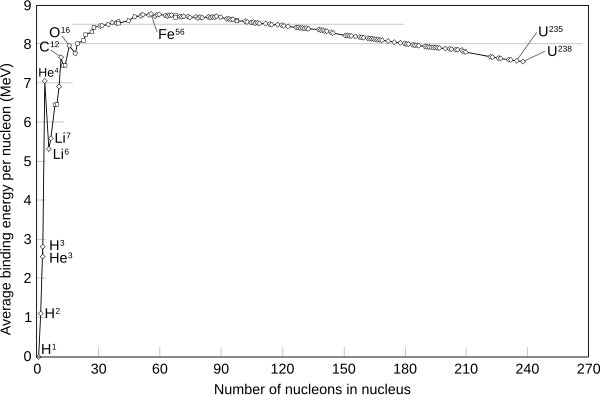
The chart shows that fusion, or combining, of hydrogen nuclei to form heavier atoms releases energy, as does fission of uranium, the breaking up of a larger nucleus into smaller parts.
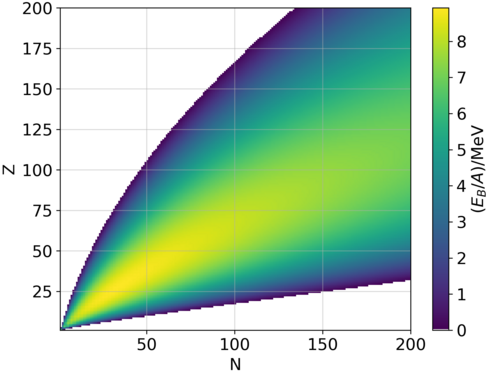
As a consequence, the listed mass deficits are not a measure of the stability or binding energy of isolated nuclei, but for the whole atoms.
Examples of this have been shown in bound-state β decay experiments performed at the GSI heavy ion accelerator.
Finally, in the heavier elements, there is a gradual decrease in binding energy per nucleon as atomic number increases.
[18] This is the approximate basic reason why iron and nickel are very common metals in planetary cores, since they are produced profusely as end products in supernovae and in the final stages of silicon burning in stars.
In fact, it has been argued that photodisintegration of 62Ni to form 56Fe may be energetically possible in an extremely hot star core, due to this beta decay conversion of neutrons to protons.
Elements with high binding energy per nucleon, like iron and nickel, cannot undergo fission, but they can theoretically undergo fusion with hydrogen, deuterium, helium, and carbon, for instance:[20] It is generally believed that iron-56 is more common than nickel isotopes in the universe for mechanistic reasons, because its unstable progenitor nickel-56 is copiously made by staged build-up of 14 helium nuclei inside supernovas, where it has no time to decay to iron before being released into the interstellar medium in a matter of a few minutes, as the supernova explodes.
The radioactive decay-powered light curve of such a process has been observed to happen in type II supernovae, such as SN 1987A.
The fact that the maximum binding energy is found in medium-sized nuclei is a consequence of the trade-off in the effects of two opposing forces that have different range characteristics.
If all nuclei of mass number A were to be allowed to be constructed of A neutrons, then 56Fe would release the most energy per nucleon, since it has a larger fraction of protons than 62Ni.