Neutron
They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes.
[12] The concept of isospin, in which the proton and neutron are viewed as two quantum states of the same particle, is used to model the interactions of nucleons by the nuclear or weak forces.
[7] The positively charged light nuclides, or "fission fragments", then repel, releasing electromagnetic potential energy.
The electron and positron produced in these reactions are historically known as beta particles, denoted β− or β+ respectively, lending the name to the decay process.
The lifetime from the bottle method is presently 877.75 s[28][29] which is 10 seconds below the value from the beam method of 887.7 s[30] A small fraction (about one per thousand) of free neutrons decay with the same products, but add an extra particle in the form of an emitted gamma ray:[31] Called a "radiative decay mode" of the neutron, the gamma ray may be thought of as resulting from an "internal bremsstrahlung" that arises from the electromagnetic interaction of the emitted beta particle with the proton.
[31] A smaller fraction (about four per million) of free neutrons decay in so-called "two-body (neutron) decays", in which a proton, electron and antineutrino are produced as usual, but the electron fails to gain the 13.6 eV necessary energy to escape the proton (the ionization energy of hydrogen), and therefore simply remains bound to it, forming a neutral hydrogen atom (one of the "two bodies").
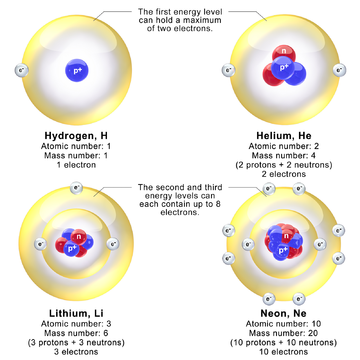
Protons and neutrons of a nuclide are organized into discrete hierarchical energy levels with unique quantum numbers.
Nucleon decay within a nucleus can occur if allowed by basic energy conservation and quantum mechanical constraints.
[48] Throughout the 1920s, physicists assumed that the atomic nucleus was composed of protons and "nuclear electrons",[51][52] but this raised obvious problems.
[59] Chadwick quickly performed a series of experiments that showed that the new radiation consisted of uncharged particles with about the same mass as the proton.
[71] In December 1938 Otto Hahn, Lise Meitner, and Fritz Strassmann discovered nuclear fission, or the fractionation of uranium nuclei into lighter elements, induced by neutron bombardment.
The energy of the gamma ray can be measured to high precision by X-ray diffraction techniques, as was first done by Bell and Elliot in 1948.
The value for the neutron's magnetic moment was first directly measured by Luis Alvarez and Felix Bloch at Berkeley, California, in 1940.
The neutron magnetic moment can be roughly computed by assuming a simple nonrelativistic, quantum mechanical wavefunction for baryons composed of three quarks.
A straightforward calculation gives fairly accurate estimates for the magnetic moments of neutrons, protons, and other baryons.
[92] The discrepancy stems from the complexity of the Standard Model for nucleons, where most of their mass originates in the gluon fields, virtual particles, and their associated energy that are essential aspects of the strong force.
[94] But the nucleon magnetic moment has been successfully computed numerically from first principles, including all of the effects mentioned and using more realistic values for the quark masses.
The Standard Model of particle physics predicts a tiny separation of positive and negative charge within the neutron leading to a permanent electric dipole moment.
New theories going beyond the Standard Model generally lead to much larger predictions for the electric dipole moment of the neutron.
[105]: 275 In 2012, Artemis Spyrou from Michigan State University and coworkers reported that they observed, for the first time, direct dineutron emission in the decay of 16Be.
The authors measured the two-neutron separation energy to be 1.35(10) MeV, in good agreement with shell model calculations, using standard interactions for this mass region.
[113] The common means of detecting a charged particle by looking for a track of ionization (such as in a cloud chamber) does not work for neutrons directly.
Within the Earth's crust a second source is neutrons produced primarily by spontaneous fission of uranium and thorium present in crustal minerals.
In addition, high-energy nuclear reactions (such as occur in cosmic radiation showers or accelerator collisions) also produce neutrons from disintegration of target nuclei.
[121] Nuclear fission reactors naturally produce free neutrons; their role is to sustain the energy-producing chain reaction.
Thermal neutrons can be polarized by transmission through magnetic materials in a method analogous to the Faraday effect for photons.
If radiation is delivered in small sessions to damage cancerous areas, normal tissue will have time to repair itself, while tumor cells often cannot.
The tumor is then bombarded with very low-energy neutrons (although often higher than thermal energy) which are captured by the boron-10 isotope in the boron, which produces an excited state of boron-11 that then decays to produce lithium-7 and an alpha particle that have sufficient energy to kill the malignant cell, but insufficient range to damage nearby cells.
The deuterium in heavy water has a very much lower absorption affinity for neutrons than does protium (normal light hydrogen).
This physical fact thus causes ordinary non-weapons grade materials to become of concern in certain nuclear proliferation discussions and treaties.


β −
radiation, the emission of a fast electron from the nucleus. The decay also creates an antineutrino (omitted) and converts a neutron to a proton within the nucleus.
The inset shows beta decay of a free neutron; an electron and antineutrino are created in this process.
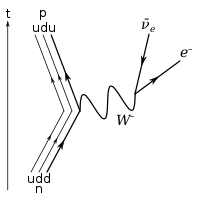
β −
decay of a neutron into a proton, electron, and electron antineutrino via an intermediate heavy
W −
boson
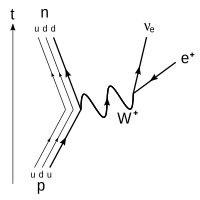
β +
decay of a proton into a neutron, positron, and electron neutrino via an intermediate heavy
W +
boson