Maxwell construction
Named for physicist James Clerk Maxwell, it considers areas of regions on phase diagrams.
[1] This basic stability requirement, and similar ones for other conjugate pairs of variables, is violated in analytic models of first order phase transitions.
This violation is not a defect, rather it is the origin of the observed discontinuity in properties that distinguish liquid from vapor, and defines a first order phase transition.
In fact, let us imagine the fluid in a state corresponding to this part of the curve contained in a heat conducting vertical cylinder whose top is formed by a piston.
The piston will, therefore, continue to move and the gas to expand until it reaches the state represented by the maximum of the isotherm.
Vice versa, if we add ever so little to the load of the balanced piston, the gas will collapse to the state corresponding to the minimum of the isotherm."
This situation is similar to a body exactly balanced at the top of a smooth surface that, with the slightest disturbance will depart from its equilibrium position and continue until it reaches a local minimum.
Their locus forms a spinodal curve which bounds a region where no homogeneous stable states can exist.
Experiments show that if the volume of a vessel containing a fixed amount of liquid is heated and expands at constant temperature, at a certain pressure,
1) bubbles nucleate so the fluid is no longer homogeneous, but rather it has become a heterogeneous mixture of boiling liquid and condensing vapor.
For many years such states were an academic curiosity; Callen[8] gave as an example, "water that has been cooled below 0°C at a pressure of 1 atm.
A tap on a beaker of water in this condition precipitates a sudden dramatic crystallization of the system."
However, studies of boiling heat transfer have made clear that metastable states occur routinely as an integral part of this process.
[10] Moreover, the fact that they are predicted by the van der Waals equation, and cubic equations in general, is compelling evidence of its efficacy in describing phase transitions; Sommerfeld described this as follows:[11]It is very remarkable that the theory due to van der Waals is in a position to predict, at least qualitatively, the existence of the unstable [called metastable here] states along the branches AA′ or BB′ [BC and FE in Fig.
Such a condition was first enunciated in a clever thermodynamic argument by Maxwell at a lecture he delivered to the British Chemical Society on Feb 18, 1875[14] (Fig.1, including the letters B C D E F, is the curve he described): The portion of the curve from C to E represents points which are essentially unstable, and which cannot therefore be realized.
Now let us suppose the medium to pass from B to F along the hypothetical curve B C D E F in a state always homogeneous, and to return along the straight line path F B in the form of a mixture of liquid and vapor.
On a temperature—molar entropy plane, the area under any curve is the heat transfer to the substance per mole, positive going from left to right and negative from right to left; moreover, in a cyclic process the net heat transfer to the substance is the area enclosed by the cycle's closed curve.
[15][16] Since the cycle Maxwell considered is composed of the two gray dashed isothermals at the same temperature, one proceeding from B to F (through C D and E), and the other directly back from F to B, the two lines are identical, just traversed in reverse; there is zero area enclosed, and hence
At the end of his lecture, after complimenting van der Waals by referring to his work as "an exceedingly ingenious thesis", Maxwell finished it by saying: I must not, however, omit to mention a most important American contribution to this part of thermodynamics by Prof. Willard Gibbs of Yale College U.S., who has given us a remarkably simple and thoroughly satisfactory method of representing the relations of the different states of matter by means of a model.
[18] This condition can be deduced by a simple physical argument as follows: the energy required to vaporize a mole is from the second law at constant temperature
However, it is the nature of the phase transition phenomenon that this inversion is not unique; for example the van der Waals quation written for
given previously between the saturated liquid and vapor states and applying the Gibbs criterion of material equilibrium to this phase change process requires writing it as
Another method to determine the coexistence points is based on the Helmholtz potential minimum principle, which states that in a system in diathermal contact with a heat reservoir
Included in this figure is the (dashed/solid) straight line that has a double (common) tangent with the curve of the function
defined earlier by Gibbs,[31][32] was originally used by van der Waals (he called it both a double and common tangent),[33] because it could be easily extended to include binary fluid mixtures for which an isotherm of
, and tabular results presented;[37][38] however, the equations also admit an analytic parametric solution that, according to Lenkner,[39] was obtained by Gibbs.
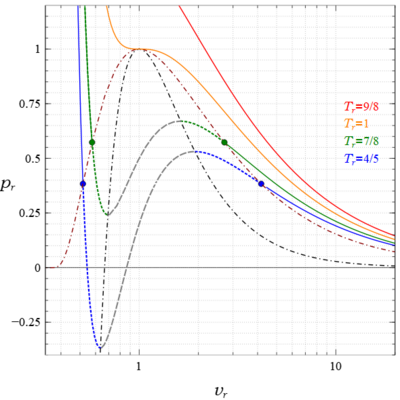
The two blue and two green circles denote the saturated liquid and vapor states on their respective isotherms.
There are also observed heterogeneous states everywhere under the coexistence curve that satisfy the lever rule; however, they are not homogeneous states of the van der Waals equation, so their existence, indicated by horizontal lines connecting the saturation points on each subcritical isotherm, is not displayed.
This means that some part of those liquid metastable states are in tension, and the lower the temperature the greater the tensile stress.
[43] Liquids that are clean and free of dissolved gas can be subjected to tensions greater in magnitude than