Phase rule
The rule was derived by American physicist Josiah Willard Gibbs in his landmark paper titled On the Equilibrium of Heterogeneous Substances, published in parts between 1875 and 1878.
Mathematically, the equation μliq(T, p) = μvap(T, p), where μ, the chemical potential, defines temperature as a function of pressure or vice versa.
The rule is valid provided the equilibrium between phases is not influenced by gravitational, electrical or magnetic forces, or by surface area, and only by temperature, pressure, and concentration.
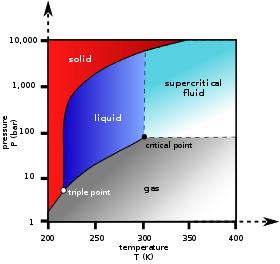
However, if the temperature and pressure combination ranges to a point where the pure component undergoes a separation into two phases (P = 2), F decreases from 2 to 1.
[6] When the system enters the two-phase region, it is no longer possible to independently control temperature and pressure.
Throughout both processes, the temperature and pressure stay in the relationship shown by this boundary curve unless one phase is entirely consumed by evaporation or condensation, or unless the critical point is reached.
Above the critical point and away from the phase boundary curve, F = 2 and the temperature and pressure can be controlled independently.
Therefore, in a single-component system, this three-phase mixture can only exist at a single temperature and pressure, which is known as a triple point.
[6] As an example, consider the system of two completely miscible liquids such as toluene and benzene, in equilibrium with their vapours.
This system may be described by a boiling-point diagram which shows the composition (mole fraction) of the two phases in equilibrium as functions of temperature (at a fixed pressure).
The quantity of each phase is given by the lever rule (expressed in the variable corresponding to the x-axis, here mole fraction).
For the analysis of fractional distillation, the two independent variables are instead considered to be liquid-phase composition (x1L) and pressure.
In that case the phase rule implies that the equilibrium temperature (boiling point) and vapour-phase composition are determined.
Each salt, in solid form, is a different phase, because each possesses a distinct crystal structure and composition.