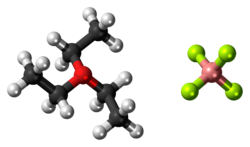
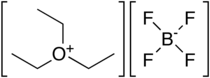Triethyloxonium tetrafluoroborate
The compounds are white solids that dissolve in polar organic solvents.
[4] Triethyloxonium tetrafluoroborate is prepared from boron trifluoride, diethyl ether, and epichlorohydrin:[5] where the Et stands for ethyl.
[7] This reagent is useful for esterification of carboxylic acids under conditions where acid-catalyzed reactions are infeasible: [8] The structure of triethyloxonium tetrafluoroborate has not been characterized by X-ray crystallography, but the structure of triethyloxonium hexafluorophosphate has been examined.
The measurements confirm that the cation is pyramidal with C-O-C angles in the range 109.4°–115.5°.
[9] Triethyloxonium tetrafluoroborate is a very strong alkylating agent, although the hazards are diminished because it is non-volatile.