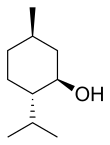
Menthol
It is a white or clear waxy crystalline substance that is solid at room temperature and melts slightly above.
For many people, menthol produces a cooling sensation when inhaled, eaten, or applied to the skin, and mint plants have been used for centuries for topical pain relief and as a food flavoring.
Menthol has local anesthetic and counterirritant qualities, and it is widely used to relieve minor throat irritation.
Natural menthol exists as one pure stereoisomer, nearly always the (1R,2S,5R) form (bottom left corner of the diagram below).
[5] In this sense, it is similar to capsaicin, the chemical responsible for the spiciness of hot chilis (which stimulates heat sensors, also without causing an actual change in temperature).
[8] Some studies show that menthol acts as a GABAA receptor positive allosteric modulator and increases GABAergic transmission in PAG neurons.
[9] Menthol has anesthetic properties similar to, though less potent than, propofol because it interacts with the same sites on the GABAA receptor.
[11] Menthol is widely used in dental care as a topical antibacterial agent, effective against several types of streptococci and lactobacilli.
[citation needed] The biosynthesis of menthol has been investigated in Mentha × piperita and the enzymes involved in have been identified and characterized.
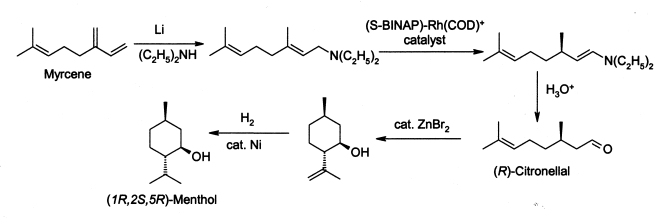
The steps of the biosynthetic pathway are as follows: Natural menthol is obtained by freezing peppermint oil.
This is cyclised by a carbonyl-ene-reaction initiated by zinc bromide to isopulegol [de], which is then hydrogenated to give pure (1R,2S,5R)-menthol.
The enantiomers are separated by chiral resolution in reaction with methyl benzoate, selective crystallisation followed by hydrolysis.
[38] Overdose effects are abdominal pain, ataxia, atrial fibrillation, bradycardia, coma, dizziness, lethargy, nausea, skin rash, tremor, vomiting, and vertigo.