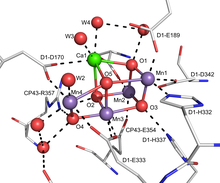
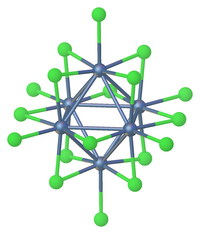
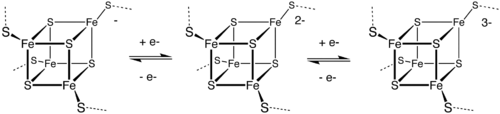
Metal cluster compound
In the 1970s, Paolo Chini demonstrated that very large clusters could be prepared from the platinum metals, one example being [Rh13(CO)24H3]2−.
In addition, cationic (positively charged) rather than neutral organometallic trimolybdenum[4][5] or tritungsten[6] clusters are also known.
F. Albert Cotton established that "ReCl3" in fact features subunits of the cluster Re3Cl9, which could be converted to a host of adducts without breaking the Re-Re bonds.
In the solid state further bridging occurs between neighbours and when this compound is dissolved in hydrochloric acid a Re3Cl123− complex forms.
Some examples have been isolated using cryptate complexes of the alkali metal cation, e.g., [Pb10]2− anion, which features a capped square antiprismatic shape.
The compound is prepared from oxidation of K4Pb9 [15] by Au+ in PPh3AuCl (by reaction of tetrachloroauric acid and triphenylphosphine) in ethylene diamine with 2.2.2-crypt.
The icosahedral tin cluster Sn122− or stannaspherene anion is another closed shell structure observed (but not isolated) with photoelectron spectroscopy.
[19] These clusters consist of at least two different (semi)metallic elements, and possess more direct metal-metal than metal-ligand contacts.
The clusters appear as discrete units in intermetallic compounds separated from each other by electropositive atoms such as [Sn@Cu12@Sn20]12−,[20] as soluble ions [As@Ni12@As20]3−[13] or as ligand-stabilized molecules such as [Mo(ZnCH3)9(ZnCp*)3].