Histidine
It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH.
The conjugate acid (protonated form) of the imidazole side chain in histidine has a pKa of approximately 6.0.
The remaining proton of the imidazole ring can reside on either nitrogen, giving rise to what are known as the N3-H or N1-H tautomers.
[9] The acid-base properties of the imidazole side chain are relevant to the catalytic mechanism of many enzymes.
[10] In catalytic triads, the basic nitrogen of histidine abstracts a proton from serine, threonine, or cysteine to activate it as a nucleophile.
The tautomerism and acid-base properties of the imidazole side chain has been characterized by 15N NMR spectroscopy.
This change indicates that the N1-H tautomer is preferred, possibly due to hydrogen bonding to the neighboring ammonium.
The shielding at N3 is substantially reduced due to the second-order paramagnetic effect, which involves a symmetry-allowed interaction between the nitrogen lone pair and the excited π* states of the aromatic ring.
Poly-histidine tags (of six or more consecutive H residues) are utilized for protein purification by binding to columns with nickel or cobalt, with micromolar affinity.
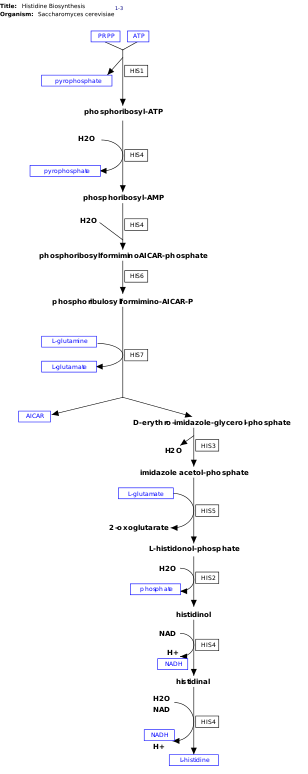
[15] Histidine is synthesized from phosphoribosyl pyrophosphate (PRPP), which is made from ribose-5-phosphate by ribose-phosphate diphosphokinase in the pentose phosphate pathway.
The first reaction of histidine biosynthesis is the condensation of PRPP and adenosine triphosphate (ATP) by the enzyme ATP-phosphoribosyl transferase.
[18] A genetic study of N. crassa histidine mutants indicated that the individual activities of the multienzyme complex occur in discrete, contiguous sections of the His-3 genetic map, suggesting that the different activities of the multienzyme complex are encoded separately from each other.
[18] However, mutants were also found that lacked all three activities simultaneously, suggesting that some mutations cause loss of function of the complex as a whole.
[22] The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002.
[29] Supplemental histidine is being investigated for use in a variety of different conditions, including neurological disorders, atopic dermatitis, metabolic syndrome, diabetes, uraemic anaemia, ulcers, inflammatory bowel diseases, malignancies, and muscle performance during strenuous exercise.