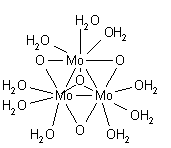
Metal ions in aqueous solution
Compounds of the metallic elements usually form simple aqua ions with the formula [M(H2O)n]z+ in low oxidation states.
A few metallic elements that are commonly found only in high oxidation states, such as niobium and tantalum, are not known to form aqua cations; near the metal–nonmetal boundary, arsenic and tellurium are only known as hydrolysed species.
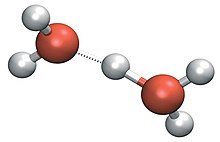
In aqueous solution the water molecules directly attached to the metal ion are said to belong to the first coordination sphere, also known as the first, or primary, solvation shell.
In fact there is a very good linear correlation between hydration enthalpy and the ratio of charge squared to ionic radius, z2/r.
Magnesium and calcium ions are common constituents of domestic water and are responsible for permanent and temporary hardness, respectively.
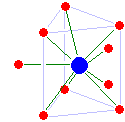
X-ray diffraction on solutions yields a radial distribution function from which the coordination number of the metal ion and metal-oxygen distance may be derived.
In a classic experiment, measurements were made on four nickel chloride solutions using the combinations of 58Ni, 60Ni, 35Cl and 37Cl isotopes to yield a very detailed picture of cation and anion solvation.
This angle is affected by the hydrogen bonds formed between water molecules in the primary and secondary solvation shells.
For example, the solvation number of 5.5 for a lithium chloride solution could be interpreted as being due to presence of two different aqua ions with equal concentrations.
For example, a solvation number of 3 for a lithium chloride solution could be interpreted as being due to the equilibrium lying wholly in favour of the ion pair.
If the frequencies are very similar it can be concluded that the coordination number of the metal ion is the same in solution as it is in a compound in the solid state.
In aqueous solution, this immediately attaches itself to a water molecule,[20] forming a species generally symbolised as H3O+ (sometimes loosely written H+).
Neutron diffraction data gave a solvation number for calcium chloride, CaCl2, which is strongly dependent on concentration: 10.0±0.6 at 1 mol·dm−3, decreasing to 6.4±0.3 at 2.8 mol·dm−3.
[31] From lanthanum to dysprosium, the coordination number is maintained at 9 with a tricapped trigonal prismatic structure, although starting from samarium the capping water molecules are no longer equally strongly bounded.
The configuration is maintained despite the small size of the cations and the water deficit, probably due to strong hydrogen bonding.
[27] Distortion occurs for low-coordinate metals with strong covalent tendencies due to the second-order Jahn-Teller effect.
[44] Quantum mechanical calculations suggests that the germanium(II) aqua ion shows extreme distortion of the first coordination sphere due to the high charge density and the stereochemically active lone pairs.
[51][52] Quantum mechanical calculations reveal a solvation number of 8, with the first coordination sphere splitting into two hydration hemispheres with 4 water molecules each.
[53] Bismuth(III) is eight-coordinate square antiprismatic in aqueous solution, though in the solid state it is nine-coordinate tricapped triangular prismatic.
This results in significant water–water hydrogen bonding and network formation already within the first hydration shell, to an extent that does not occur for cation solvation.
They can be viewed as particularly stable hydrolysis products in a hypothetical reaction such as The vanadium has a distorted octahedral environment (point group C4v) of one oxide ion and 5 water molecules.
The aqua ion UO2+2(aq) has five water molecules in the plane perpendicular to the O-U-O axis in a pentagonal bipyramid structure, point group D5h.
Most experimental measurements relate to the heat evolved when a salt dissolves in water, which gives the sum of cation and anion solvation enthalpies.
There is an excellent linear correlation between hydration enthalpy and the ratio of charge squared, z2, to M-O distance, reff.
It is notable that the heavy alkali metals have rather small entropy values which suggests that both the first and second solvation shells are somewhat indistinct.
Modeling the hydrolysis reactions that occur in solution is usually based on the determination of equilibrium constants from potentiometric (pH) titration data.
The logarithm of hydrolysis constant, K1,-1, for the removal of one proton from an aqua ion shows a linear relationship with the ratio of charge to M-O distance, z/d.
[69] This is because of the low coordination numbers of ions in this part of the periodic table (also including Ag+ and Au+), so that fewer water molecules are present around the cation and they experience more electrostatic force than normal.
Oxyanions can be viewed as the end products of hydrolysis, in which there are no water molecules attached to the metal, only oxide ions.
The most information on the kinetics a water exchange comes from systems with a residence time between about 1 μs and 1 s. The enthalpy and entropy of activation, ΔH‡ and ΔS‡ can be obtained by observing the variation of rate constant with temperature.